[ad_1]
A 4-year-old girl from Maryland is said to have mistakenly received the first dose of the COVID-19 vaccine, instead of the seasonal flu vaccine she was to receive at her local pharmacy.
According to the Baltimore Sun, Victoria Olivier got her daughter for a flu shot this month, although a Walgreens pharmacist instead administered an adult dose of the Pfizer COVID-19 vaccine, which has yet to be released. Food and Drug Administration (FDA) approved for children under 12 years old.
Walgreens spokesperson Phil Caruso told Fox News in an email that patient safety was the company’s “top priority”.
“Events like this are extremely rare and we take this matter very seriously,” Caruso wrote in a statement. “We are in contact with the patient’s family and have apologized. Our multi-step vaccination procedure includes several safety checks to minimize the risk of human error. We recently reviewed this process with our pharmacy staff to ‘avoid a future event. “
TRICK-OR-TREATING AMONG THE COVID-19 PANDEMIC: CDC DIRECTOR Weighs in
The error comes as children under 12 remain ineligible for the vaccine, although Pfizer-BioNTech has submitted clinical trial data from a study of the COVID-19 vaccine in children aged 5 to the FDA. at age 11, the companies said on Tuesday. An emergency approval request is expected to follow in the coming weeks, Fox News reported. Trial data included results among 2,268 participants aged 5 to 11, suggesting that the lower dose was safe, well tolerated, and resulted in neutralizing antibody responses.
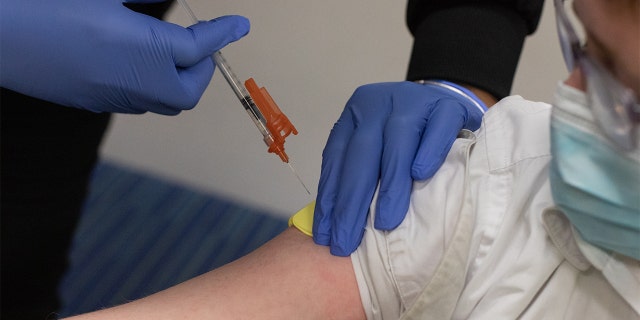
A healthcare worker administers a dose of a Pfizer-BioNTech Covid-19 vaccine to a child in a pediatrician’s office in Bingham Farms, Michigan, United States, Wednesday, May 19, 2021. Anthony Fauci, National Director Institute of Allergy and Infectious Diseases, said in an interview with MSNBC that it expects all children to be eligible for a Pfizer-BioNTech Covid-19 vaccine by the end of the year. Photographer: Emily Elconin / Bloomberg via Getty Images
(Emily Elconin / Bloomberg via Getty Images)
Olivier told the Baltimore Sun that she and her family were “stunned” by the incident. They had apparently called a 24/7 nurse’s hotline, Poison Control, and reached out to Olivier’s social media network of friends for help. So far, the child has had no major side effects, the outlet reported.
PFIZER-BIONTECH SUBMIT COVID-19 VACCINE DATA TO FDA FOR CHILDREN 5-11
In a statement to Fox News, the FDA recalled that it had not “evaluated the data relating to the safety and efficacy” of the Pfizer vaccine for use in children under the age of 12, nor approved or authorized emergency use of the vaccine for this pediatric population. .
“We are happy to hear that the child is doing well and hope that she has finally received her flu shot,” the spokesperson wrote in an email. “In accordance with the vaccine provider’s agreement, it is mandatory for immunization providers to report vaccine administration errors to VAERS, whether or not they are associated with an adverse event. [The Vaccine Adverse Event Reporting System]. “
The Olivier family apparently have no intention of filing a complaint with the Maryland Board of Pharmacy.
Kayla Rivas of Fox News contributed to this report.
[ad_2]
Source link