[ad_1]
By James Gallagher
Health and science correspondent
- Posted
-
-
Coronavirus pandemic
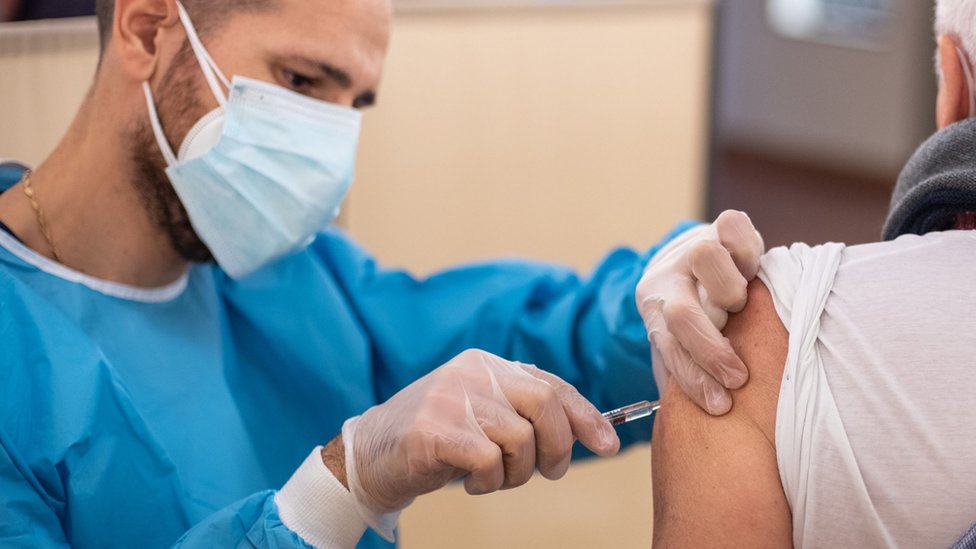
image copyrightGetty Images
The first effective coronavirus vaccine can prevent more than 90% of people from contracting Covid-19, according to preliminary analysis.
The developers – Pfizer and BioNTech – described it as “a great day for science and humanity.”
Their vaccine has been tested on 43,500 people in six countries and no safety concerns have been raised.
The companies plan to apply for an emergency clearance to use the vaccine by the end of the month.
No vaccine has gone from the drawing board to proving its effectiveness in such a short time.
There are still huge challenges ahead, but the announcement has been warmly received with scientists describing themselves as smiling “ear to ear” and some suggesting that life may return to normal in the spring.
“I’m probably the first to say it, but I’ll say it with some confidence,” said Sir John Bell, regius professor of medicine at the University of Oxford.
-
Stock markets soar on hopes of Covid vaccine
- Analysis: Do we finally have a vaccine?
- Who would receive the coronavirus vaccine and how?
How effective could this be?
A vaccine – alongside better treatments – is seen as the best way out of the restrictions that have been placed on all of our lives.
The data show that two doses, three weeks apart, are needed. Trials – in the United States, Germany, Brazil, Argentina, South Africa and Turkey – show that 90% protection is achieved seven days after the second dose.
However, the data presented is not the final analysis as it is based only on the first 94 volunteers to develop Covid, so the precise efficacy of the vaccine may change when the full results are analyzed.
Dr Albert Bourla, President of Pfizer, said: “We are an important step in providing people around the world with a much needed breakthrough to help end this global health crisis.
Professor Ugur Sahin, one of the founders of BioNTech, called the results a “milestone”.
When will the vaccine be available?
A limited number of people could receive the vaccine this year.
Pfizer and BioNTech say they will have enough safety data by the third week of November to present their vaccine to regulators.
Until it is approved, countries will not be able to start their vaccination campaigns.
The two companies say they can deliver 50 million doses by the end of this year and about 1.3 billion by the end of 2021. Each person needs two doses.
The UK is expected to receive 10 million doses by the end of the year, with 30 million more doses already on order.
Who would get it?
Not everyone will get the vaccine right away, and countries each decide which ones should be given priority.
Hospital staff and nursing home workers will top every list because of the vulnerable people they work with, as will the elderly who are most at risk for serious illness.
The UK is likely to prioritize elderly residents in nursing homes and those working there.
People under 50 and without medical problems are likely to be the last in the queue.
Are there any potential issues?
Many questions remain unanswered as these are only provisional data.
We do not know if the vaccine is preventing you from spreading the virus or simply developing symptoms. Or if it works just as well in older people at high risk.
The bigger question – how long does immunity last – will take months, if not years, to answer.
There are also enormous manufacturing and logistical challenges to vaccinate large numbers of people, as the vaccine must be stored in an ultra-cold warehouse at a temperature below minus 80 ° C.
The vaccine appears immune to major trials so far, but nothing, including paracetamol, is 100% safe.
How it works?
There are about a dozen vaccines in the final stages of testing – known as the Phase 3 trial – but this is the first to show results.
It uses a completely experimental approach – which involves injecting part of the virus’s genetic code – in order to train the immune system.
Previous trials have shown that the vaccine causes the body to make both antibodies – and another part of the immune system called T cells to fight the coronavirus.
What was the reaction?
UK Chief Medical Advisor Professor Chris Whitty said the findings showed the “power of science” and was “reason for optimism” for 2021.
The first news that the Pfizer / BioNTech vaccine is working demonstrates the power of science against COVID. We need to see the final data on safety and efficacy, but it’s very encouraging.
Continuing to suppress COVID is essential, but it’s a reason to be optimistic for 2021.
– Professor Chris Whitty (@CMO_England) November 9, 2020
US President-elect Joe Biden said this was “great news”.
“It is also important to understand that the end of the battle against Covid-19 is still months away,” he added.
The British Prime Minister’s official spokesperson said the results were “promising” and that “the NHS is ready to start a vaccination program for those most at risk once a Covid-19 vaccine becomes available” .
Professor Peter Horby, University of Oxford, said: “This news made me smile from ear to ear.
“It’s a relief … there’s a long way to go before vaccines start to make a real difference, but it feels like a watershed moment to me.”
To follow James on Twitter
View comments
Related topics
-
Coronavirus vaccine: UK signs agreements for 90 million doses of vaccine against the virus
[ad_2]
Source link