:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/LI2MZUDBDJGTMMTES7HQSW5WHA.jpg)
[ad_1]
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/D3LIQJGUXHM4FWNSXHZ7WLOTWI.jpg)
Hours after Argentina will exceed 50,000 dead for COVID-19[feminine, Infobae a confirmé que Mercredi prochain matin, 580000 doses de vaccin arriveront dans le pays Covishield, une variante de celle fabriquée par AstraZeneca / Oxford, mais produite par le Serum Institute of India, en collaboration dans le cadre d’un transfert de technologie du laboratoire anglo-suédois et de l’université britannique.
La nouvelle est d’une grande importance pour notre pays à un moment où seul le vaccin russe Spoutnik V est appliqué au personnel de santé sans avoir atteint jusqu’à présent un approvisionnement suffisamment important et fluide pour les appliquer en masse. Le gouvernement pourrait décider, dans ce qui serait une nouvelle stratégie épidémiologique, que le vaccin Covishield soit appliqué en une seule dose (stratégie un tir), les dernières analyses effectuées par le laboratoire AstraZeneca ayant prouvé qu’en une seule application le vaccin atteint 76% d’efficacité contre le nouveau coronavirus SRAS-CoV-2; un pourcentage de protection suffisant, tel qu’établi par l’Organisation mondiale de la santé (OMS) pour une inoculation virale de ce type.
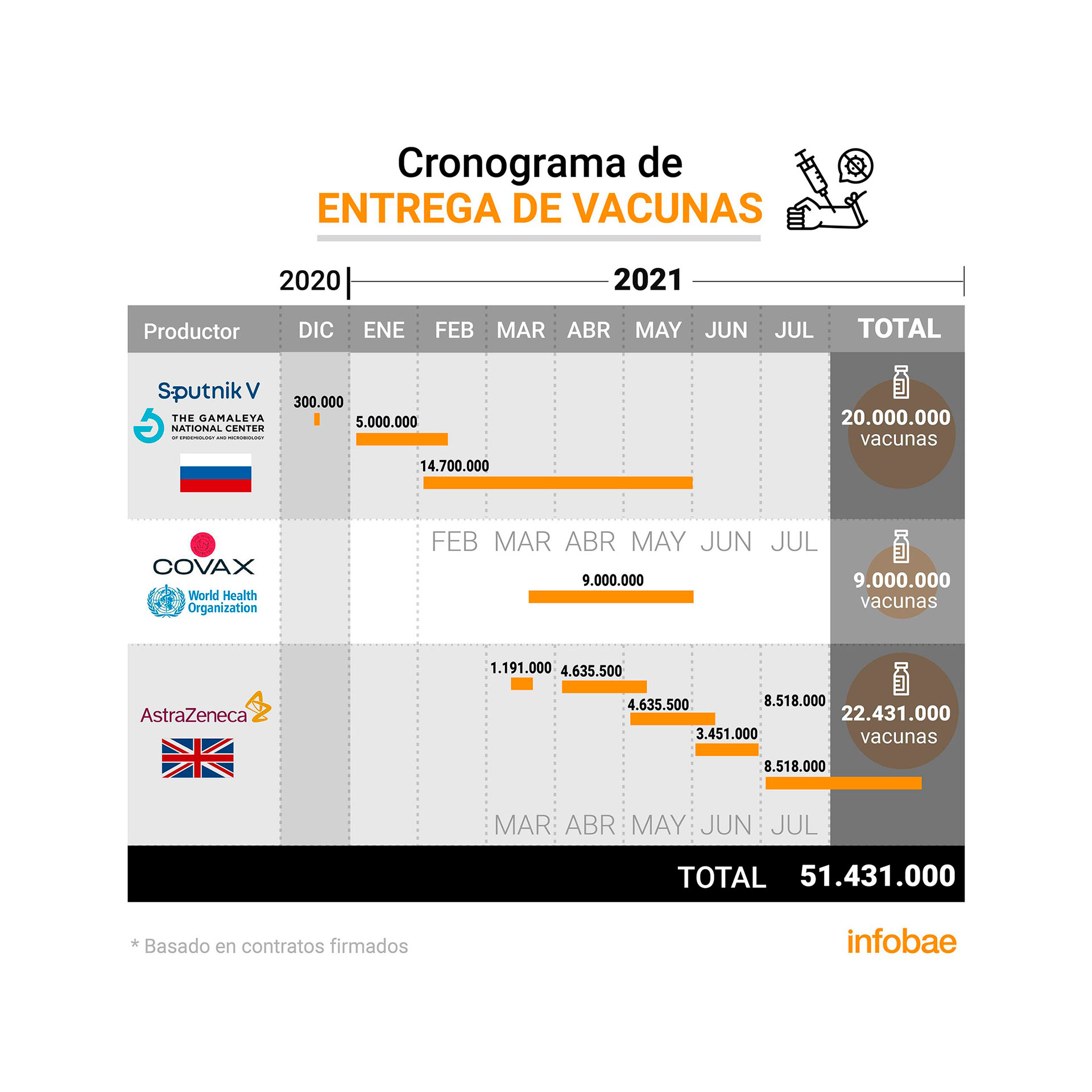
De cette manière, les autorités sanitaires évalueraient si 580 000 doses permettront de vacciner 290 000 personnes avec deux doses, ou 580 000 personnes avec une seule dose. Les sources d’AstraZeneca ont annoncé qu’en mars le pays recevrait également un deuxième lot de 580 000 doses.
Hier, après l’arrivée de la troisième livraison de vaccins Spoutnik V dans le pays, sur un nouveau vol d’Aerolineas Argentinas, le ministre de la Santé de la Nation, Ginés González García, a anticipé à ses collègues provinciaux et CABA l’arrivée des vaccins en provenance d’Inde la semaine prochaine pour renforcer la campagne de vaccination du personnel de santé et ainsi passer à la phase de vaccination des plus de 70 ans.
“La bonne nouvelle est que nous avons plus de vaccins. Aux vaccins qui arriver aujourd’hui [por ayer] -400,000 doses of Sputnik V vaccine- We must add those who arrive early next week from India to cover all health workers and start vaccinating people over 70 years old, ”explained González García during the meeting with ministers from the country’s 24 jurisdictions. , within the framework of the Federal Council of Health (Cofesa).
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/Q3HBK3QX7D36EWRKUZAB2SCCEY.jpg)
The minister celebrated that the next income from COVID-19 vaccines “they will make it possible to intensify the vaccination of priority groups ”. He also announced that the World Bank is providing additional funding of $ 250 million for direct transfers to the provinces “as part of the achievement of the goals of the Sumar program.”
On Tuesday February 9, the Ministry of Health of the Nation authorized the Covishield vaccine in “emergency” produced in India to fight COVID-19 disease, after the National Administration of Drugs, Food and Technology (Anmat) give the green light to its approval through a technical report, with the same mechanism that was used for the arrival of Sputnik V on December 23, 2020. In addition, the arrival of another batch of 580,000 doses in from India.
These doses will be added to the 23.6 million (was originally 22.4 million and 1.2 million more were added in an agreement signed in February), which the government has already contracted with the Anglo-Swedish laboratory in co-production with the local biotechnology company mAbxience and the Mexican Liomont and which is divided and packaged in Mexico, which will be distributed throughout the region since March.
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/BAPOIYJBQYO4LLSGAYOWRNEJTA.jpg)
As specified in resolution 627/2021, published in the Official Journal of Tuesday, the doses which will arrive next week are those of the “COVISHIELD / ChAdOx1nCoV-19 Corona Virus vaccine – recombinant vaccine produced by the Serum Institute of India”, in collaboration with the University of Oxford and AstraZeneca.
This is a vaccine produced by technology transfer from the AstraZeneca laboratory and the University of Oxford, whose immunizer had already been approved by Argentina on December 30. In this case, its approval was “to determine that said transfer (of technology) has no impact on the quality, safety and efficacyProduct that has already been approved by 12 countries, in addition to the Indian health authority.
The decree states that “there was no serious adverse event and no significant difference was identified in the efficacy observed in the different age groups participating in the clinical trials”.
The results demonstrated a vaccine efficacy of 76% (CI: 59% to 86%) after a first dose, with protection maintained until the second dose. With an interval between doses of 12 weeks or more, the efficacy of the vaccine increased to 82% (CI: 63%, 92%).
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/Q2R5EJOCBR5DHECFQKQNVX5NEE.jpg)
The analysis also showed the vaccine’s potential to reduce asymptomatic transmission of the virus, based on weekly swabs obtained from volunteers during the UK trial. The data showed that positive CRP readings were reduced by 67% (CI: 49%, 78%) after a single dose and by 50% (CI: 38% to 59%) after the two-dose regimen, which confirms a substantial impact on virus transmission.
The primary efficacy outcome was based on 17,177 participants accumulating 332 symptomatic cases from Phase III trials in the UK (COV002), Brazil (COV003) and South Africa (COV005), led by the University of Oxford and AstraZeneca; 201 more cases than previously.
The government has contracted for the supply of COVID-19 vaccines for some 62 million doses from different laboratories, as Minister Ginés González recently explained to a legislative committee.
The minister clarified that Argentina had agreements to receive 30 million doses of Sputnik V, 23.6 million AstraZeneca and 9 million through the Covax mechanism, an international initiative to ensure fair distribution of the media against COVID- 19. According to the data, the official said the government hopes to vaccinate all of Argentina’s population over the age of 18.
I KEEP READING:
What is Covishield and how does it work, the vaccine India launched with Oxford-AstraZeneca
Government allowed emergency use of COVID-19 vaccine produced in India
Vaccine diplomacy: India seeks to compete with China with many shipments to Asia
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos