:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/CRQJ7C6ICYNLFKXVPIN6ZIXVXA.jpg)
[ad_1]
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/DZQPHPA6FB3RTZD3VJZJ3SIDFI.jpg)
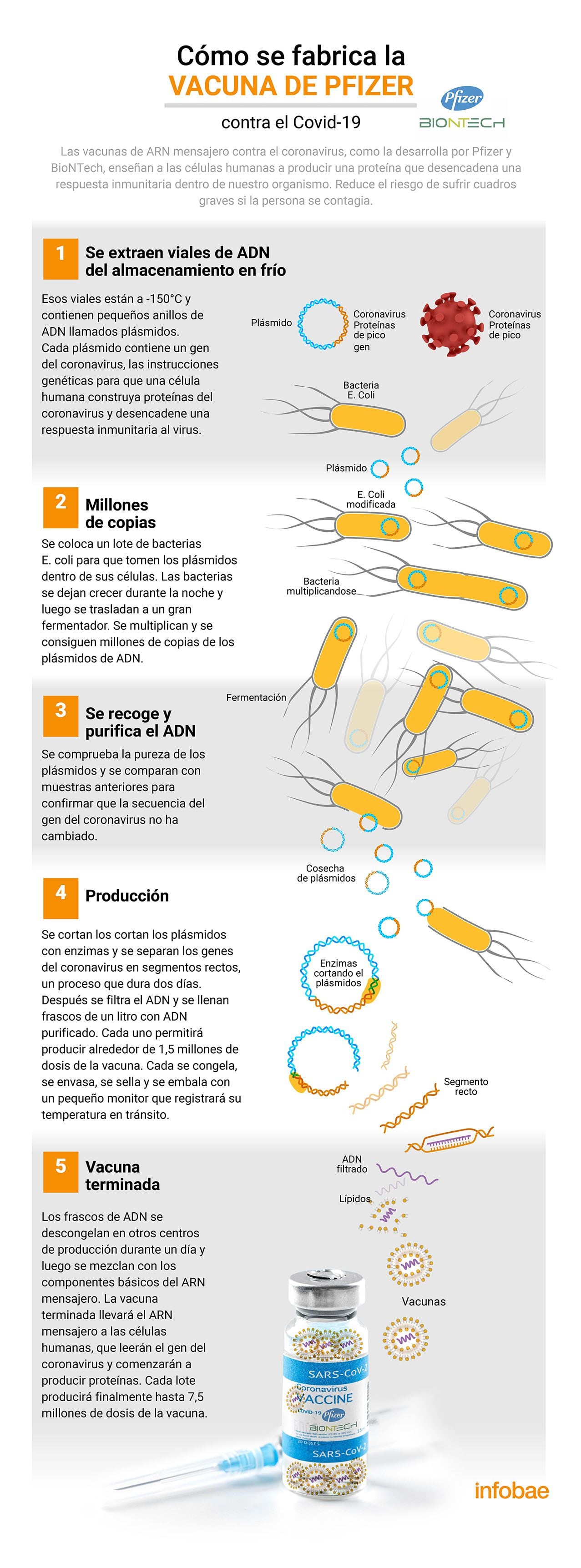
The vaccine Messenger RNA against COVID from the companies Pfizer of the United States and BioNTech of Germany is one of 20 that have already been approved for emergency use worldwide. It has an efficiency of about 91% to prevent COVID-19 disease for up to six months after inoculation of people and was the first vaccine approved by the World Health Organization (WHO) Advisory Group of Experts on Immunization. It requires 5 main steps for its development.
The process of preparing vaccine doses requires different steps that are manufactured by experts in molecular biology, computer science, chemistry, laboratory technicians, computer scientists, engineers, physicians and other specialties working in different production and storage centers in the United States and Europe.
In Latin America, the vaccine is already cleared for emergency use in Argentina, Mexico, Chile, Panama, among others. This vaccine is part of the ongoing negotiations between the Argentine government and the Pfizer company to obtain more doses for the national immunization plan.
The development of the vaccine is based on a platform on which the company BioNTech had worked to produce vaccines against other infectious diseases. And the knowledge about the special features of the coronavirus infection has been added. The virus contains proteins that it uses to enter human cells. These proteins, called spike (or “spike” in English), they are the target of vaccines and treatments. In the case of Pfizer and BioNTech products, messenger RNA is used, which is the genetic material that human cells read to make proteins.
Not
To calculate the doses, the first step is extract the DNA vials from the master cell bank, which is the source of each batch of Pfizer’s Covid-19 vaccine. Vials are stored at -150 ° C (-238 ° F) or colder and contain small DNA rings called “plasmids”.. There are instructions for human cells to build coronavirus proteins and trigger an immune response to the virus.
So, in a second time, production teams thaw plasmids and edit batches with E. coli bacteria so that they take the plasmids in their cells. These cells are grown and multiplied overnight, and the next day they are transferred to a fermenter containing up to 300 liters of nutrient broth for four days.
After this time, In a third step, DNA is collected and purified, and chemicals are added to open up the bacteria and release the plasmids from the cells that contain them. The mixture is purified to eliminate bacteria and leave only plasmids. They are compared to previous samples to confirm that the coronavirus gene sequence has not changed as a quality control.
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/CRQJ7C6ICYNLFKXVPIN6ZIXVXA.jpg)
The process continues in the fourth step which incorporates proteins called enzymes. They are responsible for cutting circular plasmids and separating coronavirus genes into straight segments. Bacterial debris or plasmid fragments are filtered and one liter vials are filled with purified DNA. It all follows that each vial of DNA is frozen, placed in a container, sealed and packaged with a small monitor that records the temperature during transport.
The last step – the fifth – consists of transcribe DNA into messenger RNA. The packaging is thawed in other production plants and then mixed with the basic components of messenger RNA. Over several hours, enzymes open DNA patterns and transcribe them into strands of messenger RNA. The finished vaccine will transport the messenger RNA to human cells, which will read the coronavirus gene and start making proteins. The mixing results in batches of vaccines.
After the production process, each dose of Pfizer and BioNTech vaccine is made up of messenger RNA wrapped in a layer that facilitates its transport and prevents damage. That is, the vaccine does not contain the virus and that means it cannot cause coronavirus infection. It also cannot modify the DNA of the person receiving it, as explained by the US Centers for Disease Control and Prevention (CDC). They released a study on Wednesday which found that Those who have been vaccinated with two doses of Moderna’s Pfizer or COVID-19 vaccine – the two most widely used in the United States – have a 94% chance of not being hospitalized because of the disease.
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/FXNTCVLCTERC3564AKPFAPGWQA.jpg)
When applied to a person, the vaccine’s messenger RNA teaches cells how to make copies of the spike protein. This way, if the person is exposed to the virus and has already received the vaccine, their body will recognize it and can better control the infection.
Consulted by Infobae, President of the Argentine Vaccination Society, Florencia Cahn, said the vaccine developed by Pfizer and BioNTech involves a production process that allows many doses to be produced in less time compared to other products. According to published studies, it is known to be more than 95% effective in preventing symptomatic forms. For more serious images, the efficiency reaches almost 100%. One drawback that it has is that it needs storage at less than 180 degrees which makes the logistics of its distribution more complex ”.
KEEP READING:
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos