[ad_1]
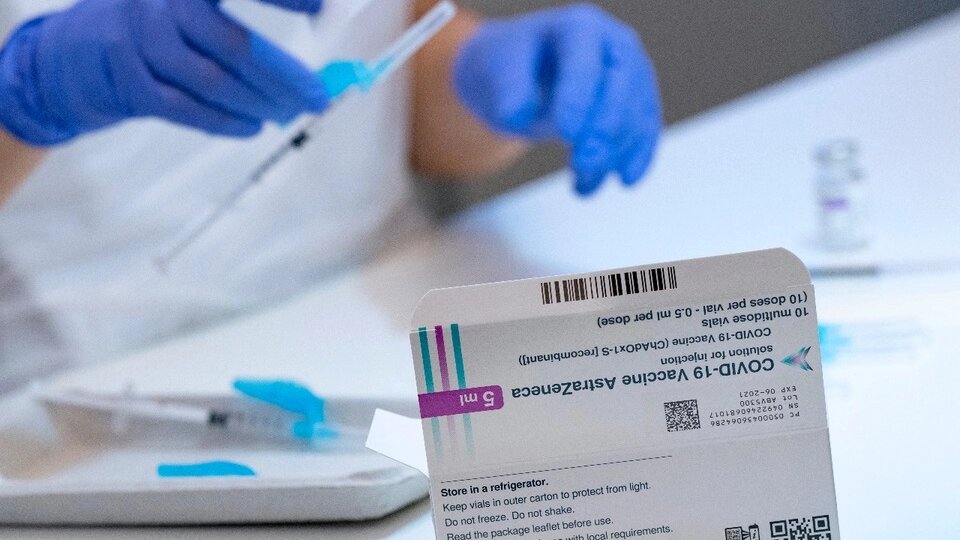
AstraZeneca has provided updated data from its clinical trials to US health authorities and reported that your coronavirus vaccine is 76% effective against symptomatic forms of the disease. Earlier this week, the Anglo-Swedish laboratory announced that the effectiveness of the drug was 79% and that it did not increase the risk of clots.
The results of “the primary analysis of phase III (vaccine) trials in the United States confirmed that (its) effectiveness was consistent “with the data announced on MondayAstraZeneca said in a statement released Wednesday.
The lab also reported that their vaccine was 100 percent effective in preventing severe cases of covid-19, a figure similar to that previously announced. Clinical trials were conducted with volunteers in Chile, Peru and the United States.
AstraZeneca pledged Tuesday to provide recent figures to the US regulator overseeing these clinical studies within 48 hours, after the The National Institute of Allergies and Infectious Diseases (NIAID) accuses the laboratory of having provided potentially “obsolete” data on its anti-covid vaccine, which would result in an “incomplete estimate of the effectiveness” of the drug.
In September 2020, the United States government launched a plan to support the development of the Covid-19 vaccine and also placed an order for 300 million doses from AstraZeneca. But so far US health authorities have not approved the emergency use of the vaccine from the Anglo-Swedish laboratory, as they did with those of Pfizer / BioNTech, Moderna and Johnson & Johnson.
This month, several countries suspended use of the AstraZeneca vaccine over fears it could cause blood clots. Last Thursday, the European Medicines Agency (EMA), however, deemed the vaccine “safe and effective” and its use has been resumed in some countries.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos