[ad_1]
Brazil on Sunday authorized the emergency use of vaccines from Chinese laboratory Sinovac and Anglo-Swedish AstraZeneca, and vaccinated the first people in a symbolic act and controversial, because fueled the political dispute between President Jair Bolsonaro and San Pablo Governor Joao Doria.
Unanimously, the five members of the collegial direction of the National Agency for Health Surveillance (Anvisa, regulator) authorized the emergency use of the two antidotes, after a five-hour meeting, broadcast live.
Although both already have Anvisa clearance for emergency use, the Sinovac vaccine developed in collaboration with the Brazilian Butantan Institute is the only viable vaccination option to immediately start the vaccination campaign in Brazil.
However, its application was conditional on the signing and publication in the Official Journal of a term of engagement by the Butantan Institute, which produces the Chinese antidote “Coronavac” in Brazil.
The condition imposed for the application of the Chinese vaccine did not stop the governor of Sao Paulo, Bolsonaro’s main political rival and who as soon as he knew the vaccine was approved, organized an event to mark the start of the vaccination in the country.
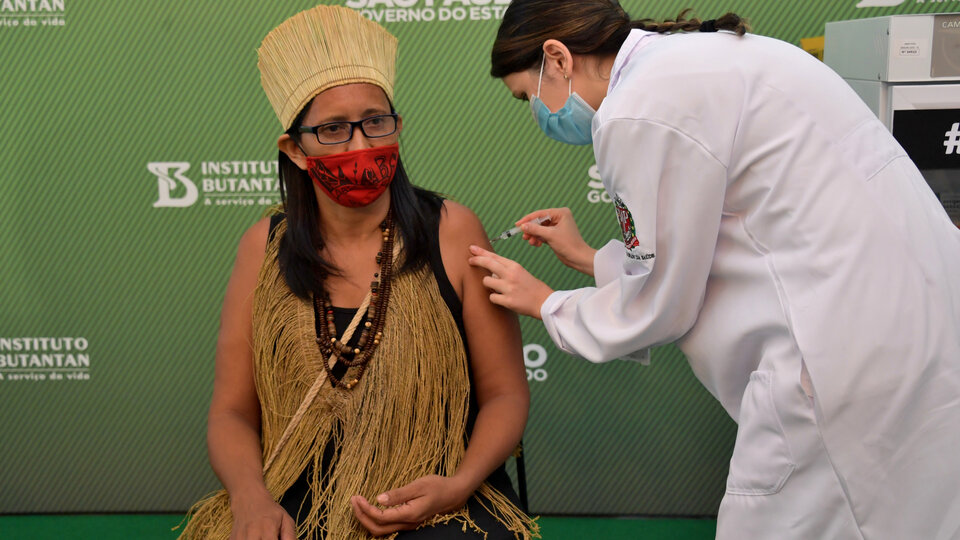
The covid-19 vaccine was first applied in Brazil to a 54-year-old black nurse, in a symbolic act that fueled the fierce conflict between Doria and the head of state.
The governor of San Pablo, who was initially a political ally of Bolsonaro, has become his main conservative rival as he has shown his interest in running for the next presidential elections in 2022, in which the president aspires to be re-elected.
From that point on, the confrontation between the two began and became more acute with the covid, as Bolsonaro is a denier of the pandemic, which he has come to call the “little flu,” while Doria has used every mechanism to deal with the virus.
The Bolsonaro government wanted to launch the national vaccination plan with the AstraZeneca vaccine, a laboratory from which it has purchased 100 million doses, but the first have not yet reached the country.
He also vetoed the purchase of the Chinese antidote, only because the management was carried out by Doria, which purchased 46 doses of the vaccine already produced in Brazil by Butantan, an entity attached to the government of São Paulo.
In the act of applying the first vaccine in São Paulo – carried out without having officially launched the national vaccination campaign -, Doria sharply criticized Bolsonaro, who was not at the event.
“It’s a triumph of science, a triumph of life against the denialists, against those who prefer the smell of death to courage and the joy of living, ”he said in his speech.
The act promoted by the governor of São Paulo has also been harshly questioned by the Minister of Health, General Eduardo Pazuello, who described it as an event of “political marketing” which will have to “solve justice”.
Pazuello recalled at a press conference that the government, in addition to the acquired doses of AstraZeneca, has purchased another 100 Chinese vaccines, which are “exclusively under contract with the Ministry of Health and the National Immunization Plan (PNI)”.
The Minister of Health reaffirmed this Sunday that after the approval of Anvisa, Brazil, with its 210,000 million inhabitants, will launch on Wednesday what he called “the biggest vaccination campaign” against the covid in the world.
“The first step has been taken to launch the world’s largest coronavirus vaccination campaign,” said Pazuello, celebrating Anvisa’s decision and stressing that Brazil has a consolidated vaccination program that serves the whole of the population, capable of immunizing up to one million people per day.
Pazuello said the health ministry will begin distributing the six million doses of antidotes the country already has available on military planes on Monday, so that the country’s 27 states can begin vaccination simultaneously throughout Brazil on Wednesday and declared that “any movement outside this line is against the law”.
Chinese “Coronavac” is 50.4% effective and was developed by the Sinovac laboratory and the Butantan Institute, one of the most prestigious medical research centers in Brazil., while the antidote developed by AstraZeneca and the University of Oxford is 70% effective.
The two antidotes have completed all three phases of testing in Brazil, one of the main requirements Anvisa demands to allow emergency use of a vaccine.
Currently, in Brazil, there are only 6 million doses of the Chinese vaccine ready for application, and five more at the doors of the Butantan oven, while there is no Oxford vaccine in the country.
The Oswaldo Cruz Foundation (Fiocruz), the main medical research center in Latin America, will this year produce 210 million doses, half of which with its own supplies, thanks to technology transfer from AstraZeneca.
As the Bolsonaro government wanted to kick off the plan with the Oxford vaccine, it bought an Indian lab for two million ready-made doses that it planned to have in the country the day before, but which would ultimately only arrive in the country within next days.
The announcement of the launch of the vaccination campaign comes as the second wave of the pandemic explodes, with half the country on high alert due to the increase in deaths and infections, and with the collapse of Amazonas state due to lack of oxygen and crowded hospitals.
With around 210,000 deaths, Brazil is the second country in the world with the highest number of deaths, behind the United States, and the third country with the highest number of infected people (8.4 million), after the country of North America and India.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos