:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/RJUCFK656RFNBOADRFA3GFKAFE.jpg)
[ad_1]
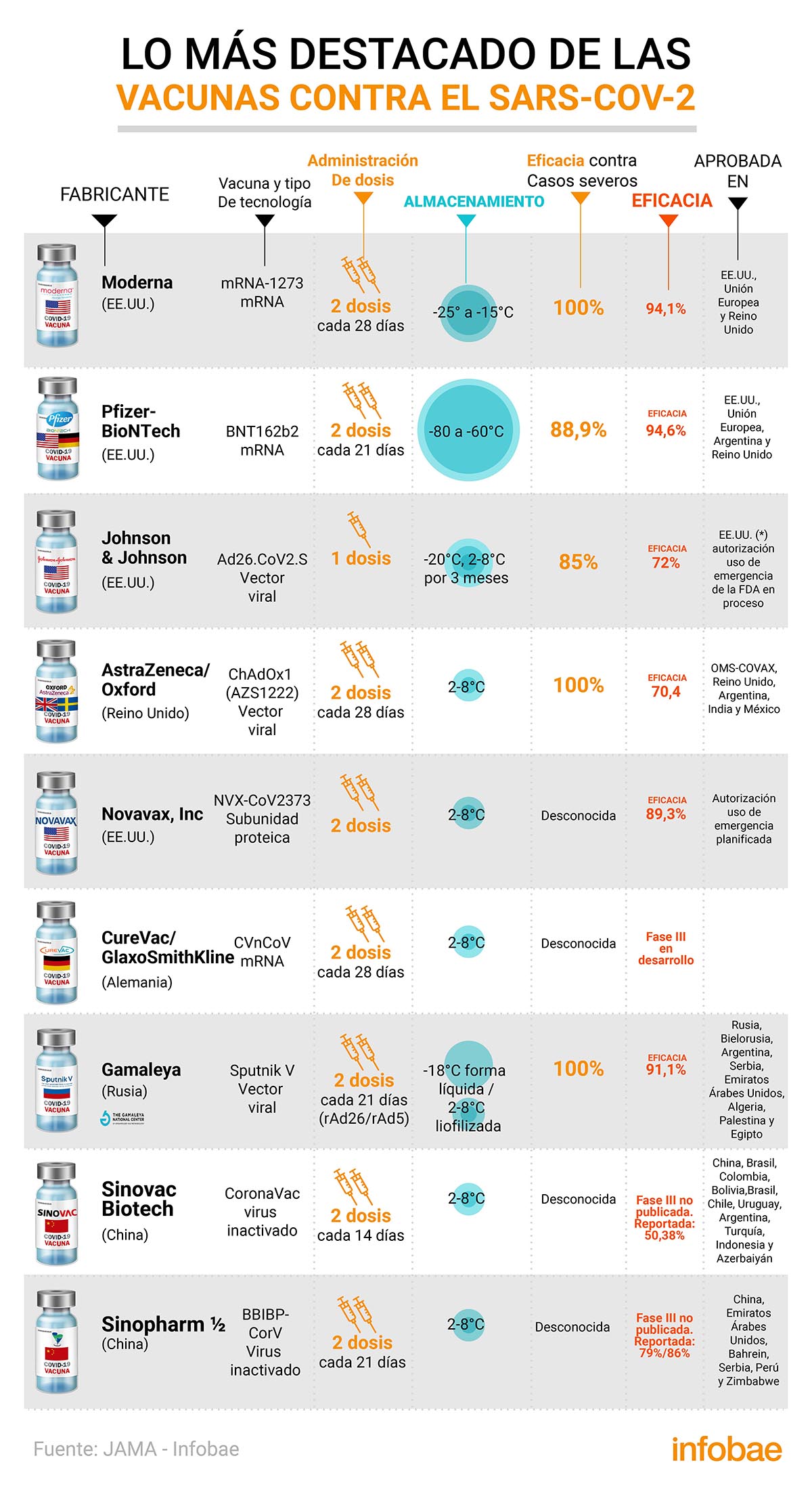
A revealing scientific article from the JAMA Network explored the key features of the 9 vaccines that have been cleared for limited emergency use against COVID-19. 5 of them, according to the famous American cardiologist Eric Topol, are distinguished by 100% protection against death and hospitalization due to the coronavirus.
As the authors noted, the researchers Buddy Creech, Shannon Walker and Robert Samuels -all from the Vanderbilt Vaccine Research Program, Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee, USA-, shortly after the emergence of SARS-CoV, at the start of the 21st century, the protein peak (S), particularly in its native prefusion conformation, has been identified as the immunodominant antigen of the virus.
Assessment of SARS-CoV-2 patients revealed that binding and neutralizing antibodies primarily target the “S1 subunit” receptor binding domain.. Once this putative vaccine target was identified, the next challenge was how to best generate an effective immune response against SARS-CoV-2. The characteristics of this response would include the production of neutralizing antibodies, the generation of a T cell response and the prevention of disease enhanced by the immune system., that is, the vaccine-induced response that led to a paradoxical increase in disease severity upon viral exposure.
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/CUEJBTPUNZAOBO3LZ6YMFGJ5IQ.JPG)
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/NF4RE42APFG6ZC2DUNBL7SXEVI.JPG)
Various expert groups have evaluated various vaccine designs when developing an inoculant against SARS-CoV-2. About these promising results in which 100% efficacy observed in five inoculants with regard to coronavirus deaths and hospitalizations, the famous American geneticist cardiologist, digital medicine researcher and author Eric Topol, said on Twitter that “¡are impressive, 100% protection against death and hospitalization in the phase III trials of the vaccines Johnson & Johnson, Pfizer, Moderna, Novanax and Sputnik V.! “, At the same time that he declared that the JAMA document “provides additional information for other vaccines in development, antigen, storage, dose, adjuvants“.
Born in 1954, Topol was president of cardiovascular medicine at the Cleveland Clinic (1991-2005) and founded the Cleveland Clinic Lerner College of Medicine. The scientist is also the founder and director of the Scripps Translational Science Institute in La Jolla, California. In turn, he is the Academic Director of Scripps Health, Professor of Genomics at the Scripps Research Center, and Senior Consultant in the Cardiovascular Disease Department at the Scripps Clinic. He is editor-in-chief of Medscape and of theheart.org.
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/PRGEFKUSUKDBW3N35XPMPMVI3Q.jpg)
The COVID-19 inoculants currently licensed for emergency use, and others for which advanced clinical data are available, are summarized in the following groups:
Inactivated and subunit protein vaccines
One approach to vaccine development is the creation of inactivated vaccines derived from viruses grown in culture and then chemically inactivated, which can provide conformationally stably expressed native antigenic epitopes. Sinopharm and Sinovac are among the most advanced manufacturers in the development of this type of vaccine, which have been evaluated by phase III trials which have obtained international authorizations for use.
Another approach to vaccine development is the administration of protein S as a recombinant protein subunit in one of the many cell-based systems that support expression of the protein. This approach can protect immunized animals in vivo, but presents the theoretical risk of generating a polarized immune response which can be overcome, depending on the adjuvant used. Novavax recently reported on its late phase clinical trials in the UK, demonstrating an efficacy of the COVID-19 vaccine of 89%. Over 60% of vaccines currently in development use a protein subunit approach, although none are licensed for use.
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/CIRLTTBO2NCGZGGK3YORKBIDJI.jpg)
Viral vector vaccines
Viral vector vaccines use replication-deficient viruses designed to express the genetic sequence of the antigen of interest in host cells. Replication-incompetent adenoviruses have been developed for HIV, tuberculosis, malaria and Ebola virus. This vaccination approach has met with varying success, often limited by pre-existing immunity to the adenovirus vector. Using adenoviruses that have minimal pre-existing immunity in the United States and Europe, 2 vaccines have shown promise early on: the adenovirus vector vaccine serotype 26 (Ad26.CoV2.S; Johnson & Johnson) and the chimpanzee adenovirus vector (ChAdOx; AstraZeneca). Both appear to be effective in preventing hospitalizations and deaths from COVID-19, but have varying effectiveness in preventing clinical illness, particularly illnesses caused by newer variants of SARS-CoV-2.
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/COKVUYMXQRFQXBJZ3BXYSVOGGU.jpg)
ARNM vaccines
New advances that take advantage of mRNA for vaccine delivery have the potential to dramatically improve vaccine development for many pathogens. In these vaccines, Lipid nanoparticles are used to protect mRNA that encodes protein S stabilized by pre-fusion on its way to intracellular space. The host uses the mRNA to produce the target protein (S protein in this case), which induces a coordinated immune response. Pfizer-BioNTech and Moderna have developed mRNA-based vaccines that demonstrate greater than 90% efficacy against SARS-CoV-2 clinical disease in clinical trials. This high vaccine efficacy is associated with very few adverse events, although local and systemic reactogenicity of the vaccine is common. The advantages of this approach are numerous, notably the speed of manufacture of the vaccine in weeks. Studies are underway or planned to assess the efficacy of currently licensed vaccines in children and against common variants of SARS-CoV-2, and to assess whether repeat vaccines containing mRNA encoding the variants may be effective. .
:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/P7GS2ANPQ6LIJUP2JIGDZG2AZI.jpg)
Vaccines are available, what’s the next step?
Once vaccines became available, barriers to administration included insufficient initial supply, inefficiencies in vaccine delivery, and a widespread vaccination surge. These obstacles have limited the ability to immunize a sufficient number of the population to achieve some measure of population immunity. Low- and middle-income countries outside of the United States have struggled to get even a minimum number of doses of the vaccine.
Slower-than-expected vaccine launch raises two important public health concerns. The first is whether it is better to ensure maximum coverage by vaccinating as many people as possible with 1 dose (2-dose vaccines) or to ensure maximum protection by strategically reserving the doses that will be used for the dose. second dose. According to US Food and Drug Administration briefing papers submitted for emergency use authorization, Moderna vaccine is over 80% effective 2 weeks after first dose and Pfizer-BioNTech vaccine is more than 50% effective after the first dose.. Second, optimizing vaccination strategies in people previously infected with SARS-CoV-2 offers another opportunity to save doses.
“Vaccination is the most important strategy to end the pandemic”warn the authors of the JAMA article. However, the emergence of multiple variants of SARS-CoV-2 with reduced susceptibility to vaccine-induced immunity and disease threatens progression. Despite these persistent threats, the efficacy of SARS-CoV-2 vaccines offers real hope for 2021.
Infographic: Marcelo Regalado
I KEEP READING:
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos