
[ad_1]
“Laboratories have been contacted to acquire the necessary doses, with the authorization of Angat. The government wants to cover the population over 18 as much as possible. Córdoba wants to buy doses added to those of the Nation and prepares the largest vaccination plan in history“, Outfit.
In this sense, he clarified that the provincial administration is working on the logistics for the vaccines of Pfizer, Sputnik V and that of AstraZeneca, and noted that they will be implemented only after the approval of Angat.
“Pfizer is the most complex, as it requires a cold chain of -80 degrees. The Sputnik V, at -18 degrees, also requires significant logistics for distribution. The one that is best suited for use is that of AstraZeneca or Oxford, from 2 to 8 degrees, “he said.
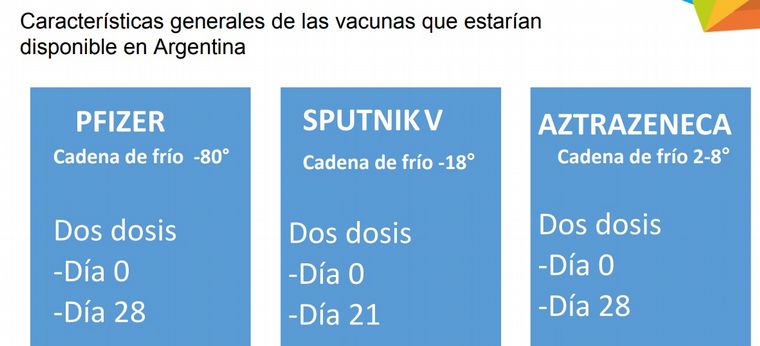
The three vaccines have two application doses: those of Pfizer and AstraZeneca on days 0 and 28, and that of Sputnik V, on days 0 and 21.
“The minimum to vaccinate in Cordoba is 1,100,000 people, and we intend to double. Initially, it will be people over 60 years old, under this age but with comorbidities, risk groups, the health and safety team and other lineages exposed to the pathology ”, explained Cardozo. .
/ Embedded code reception // End of embed code /
The process and phases of vaccine research
In this context, the process of each of the research and approval phases during which vaccines are developed has been detailed.
It is, in a first instance of a preclinical test –With cell and animal tests for an immune response-; then the phase one or safety test and dose, which is done to a small group of healthy people and on purpose; continue with phase two or extended trial -It is given to hundreds of people divided into groups for the purpose of evaluating the ability to produce antibodies and defense against the vaccine, in an advanced safety test and ability to stimulate the immune system.
In the phase three or efficacy test it is administered to thousands of people and its effectiveness is tested by contagion. These are trials large enough to reveal evidence of relatively rare side effects that might be missed in previous studies.
Finally, in the phase four or post approvalOnce the vaccine is approved, a real-world study is conducted to verify its efficacy, safety and detection of rare side effects.
Guillermo Panero Report
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos