[ad_1]
Despite his firm stand against the Cuban administration, the Washington post highlighted the scientific advances that exist on the island and the development of vaccines and the coronavirus treatments it performs. Currently, Cuba has five vaccine projects, including three designed by the Center for Genetic Engineering and Biotechnology (CIGB), which are the Abdala and Mambisa, and three developed by the Finlay Vaccine Institute (IFV), Soberana 01, Sovereign 02 and Sovereign 01-A. As published by Página / 12, Argentina is currently negotiating the arrival of Soberena 2 from May.
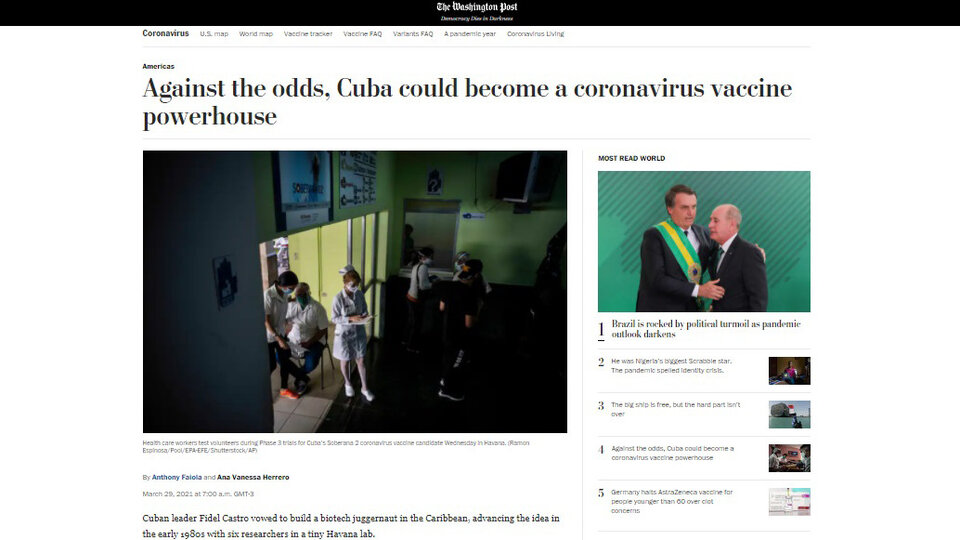
“Cuban leader Fidel Castro promised to build a biotechnology giant in the Caribbean and came up with the idea in the early 1980s with six researchers in a small Havana lab. Forty years later Communist island nation could be on the cusp of a singular breakthrough: become the smallest country in the world to develop not one, but several vaccines against the coronavirus, ”noted the American newspaper.
After clarifying its editorial line, stressing that< Cuba est un État autoritaire à parti unique avec de strictes restrictions à la liberté d'expression, à l'activisme politique et aux libertés économiques >>, the newspaper acknowledged that “investments in education and health care laid the foundation for what it is today an exceptionally sophisticated biotech device for a small developing country, with at least 31 research companies and 62 factories with over 20,000 workers“.
In its note, the Washington Post highlights the progress of Cuban science in the development of vaccines against the coronavirus and recalls that the two most advanced formulas studied in the country are Sovereign 2 and Abdala, which require two to three doses and which have a high percentage of efficiency.
If the Phase 3 trials are positive, Cuban authorities said this week, they would move on to a large ‘intervention study’ that would inoculate nearly everyone in Havana, or 1.7 million people, by here. may. By August, its goal would be to reach 60 percent of the national population, and the rest would receive doses by the end of the year, ”reports the American newspaper.
In the article, they also point out that the success of these vaccines could place Cuba “among the first nations in the world to obtain collective immunity”. The names of its most advanced vaccines, Sovereign and Abdala. taken from a poem by the hero of Cuban independence José Martí, they seem to intend to move Cuban hearts and minds, “concludes the text, which never ceases to insist that the island finally obtain” more propaganda “in case of reaching your goal.
Sovereign 2
At the beginning of March, the Cuban authorities authorized the start of the third phase clinical trials of Soberana 02, the first vaccine candidate in Latin America and the Caribbean to pass Phase II studies.
Sovereign 02, in testing since October 19, is a conjugate vaccine in which virus antigen and tetanus toxoid are combined. With this formula, immunity should reach the lining of the respiratory tract in order to prevent the entry of the virus.
Iran has agreed to host the phase 3 Sovereign trial, as part of a technology transfer deal that could produce millions of doses in that country.
According to the director of the Finlay Institute, this will be the candidate to be proposed for application in the pediatric population.
Abdala
On March 20, the Regulatory Authority for Medicines, Equipment and Medical Devices of the Republic of Cuba (CECMED) authorized the transition to phase III of the Abdala vaccine.
With this authorization, the Cuban Biotechnology and Pharmaceutical Industries Business Group (BioCubaFarma) will begin inoculating 48,000 volunteers aged 19 to 80. The tests will be carried out in the eastern provinces of Granma, Santiago de Cuba and Guantánamo.
Negotiations between Cuba and Argentina
As this newspaper reported, an agreement between the island and the Argentine government could be imminent. This Tuesday, Cuba officially delivered all the documentation on Sovereign 02, the vaccine that could be received in May. It is quite possible that this negotiation will be advanced and the doses will be available in about six to seven weeks.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos