[ad_1]
Three months after the first announcement on the advances of the Russian vaccine Sputnik V, the World Health Organization announced that it had started discussions with the Gamaleya institute incorporate it into an emergency use list, which would mean recommending the use of the vaccine by the United Nations health agency for all member states.
For now, the WHO has continued to be cautious about the Russian project. “We look forward to receiving data on its Sputnik V vaccine candidate. If a product submitted for evaluation meets the listing criteria, WHO will release the results,” the agency said in a statement.
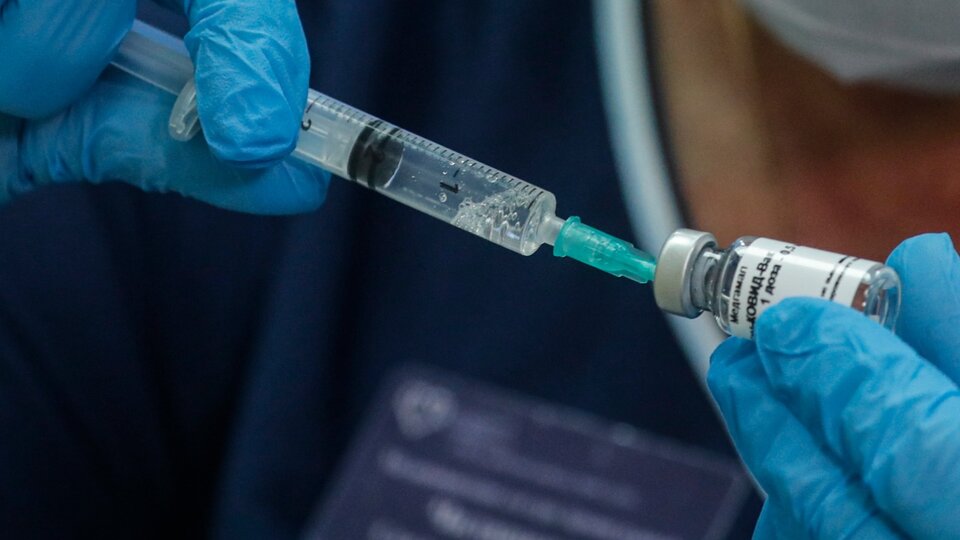
According to the results of phase 3 announced this week by the Russian government, the Russian vaccine Sputnik V is 92% effective in protecting people against COVID-19. The announcement came a day after North American laboratory Pzifer announced that preliminary testing of its development had yielded 90% effectiveness.
The development of Sputnik V uses particles created from an adenovirus, a pathogen that commonly causes the common cold in humans. The virus has been modified to carry the genes for the protein (Spike, “S”) that covers Sars CoV-2 and functions as a gateway to cells in the body.
The National Center for Epidemiology and Microbiology of Gamaleya, which has started conversations with the WHO to get approval and enter the list of emergency uses, is a century-old organization, established in 1891, where several vaccines have were designed, among the last, to fight against Ebola.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos