[ad_1]
The national government has authorized the emergency use of a vaccine made in India, after having been approved in the last hours by the National Administration of Medicines, Food and Medical Technology (ANMAT). It did so through resolution 627/2021, published this Tuesday in the Official Journal, which is signed by the Minister of Health, Ginés González García.
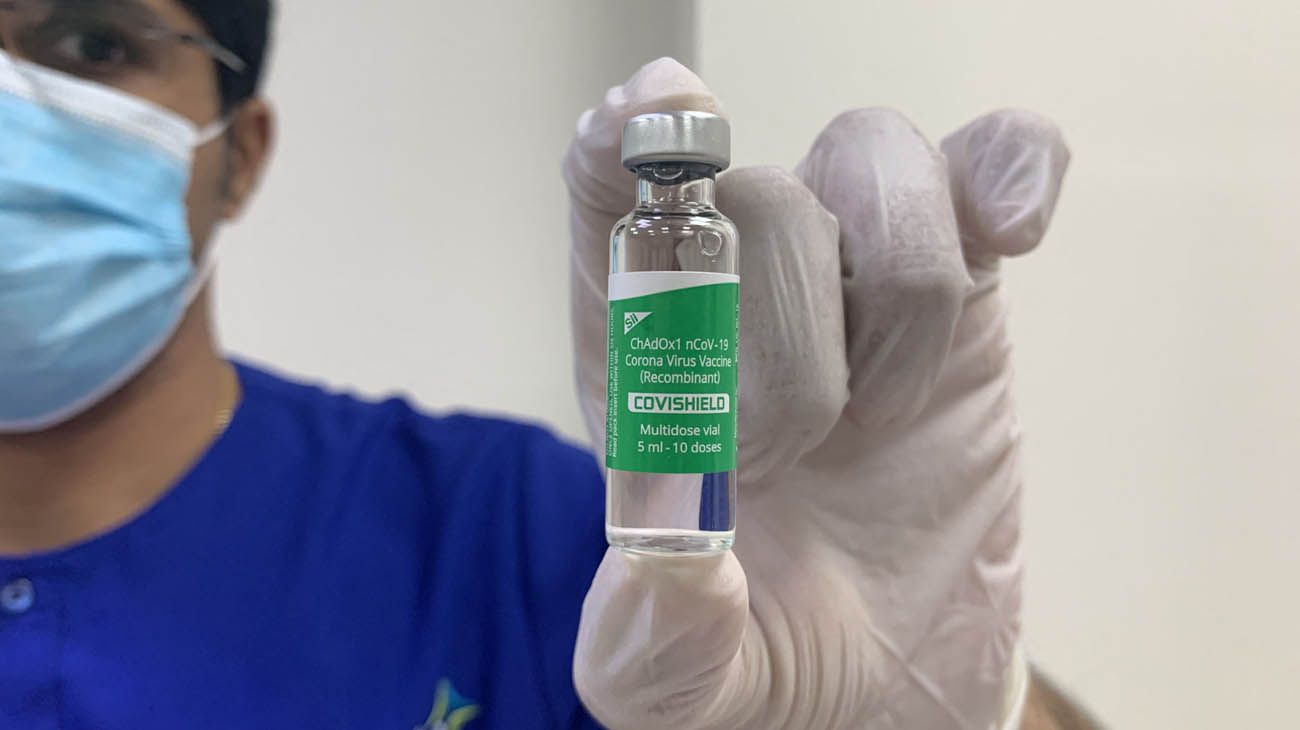
The emergency-approved coronavirus compound is produced by the Serum Institute of the Republic of India, one of the largest laboratories in Southeast Asia. The vaccine, with the name Corona Covishield / ChAdOx1nCoV-19 virus vaccine – Recombinant, is the result of a technology transfer agreement between the Serum Institute of India, the University of Oxford and the AstraZeneca laboratory.
“The vaccine against the Corona Covishield / ChAdOx1nCoV-19 virus – recombinant vaccine produced by the Serum Institute of India is authorized under Articles 8 and 9 of Law No. 27,573 and in accordance with the recommendations of the ANMAT”, establishes the Official newspaper.
India: fire at the headquarters of the world’s largest vaccine manufacturer
The document emphasizes that ANMAT has received all the information and points out that the new vaccine has been approved in its testing phase “no serious adverse events were reported and no significant differences were identified in the efficacy observed in the different age groups participating in the clinical trials.“.
The resolution indicates that the Health Quality Secretariat like the Health Access Secretariat, both of the Nation, they agreed “To this extent within the scope of its powers”.
AstraZeneca vaccine issues that concern the world
The Ministry of Health estimated that this transfer “has no impact on quality, safety and efficiency” which have already been evaluated by ANMAT during the approval of the specialty developed by the University of Oxford and AstraZeneca.
To establish its emergency use, the ANMAT “had all the information established by the emergency authorization procedure, in relation to the compliance with standards required of processing plants, development and manufacture of products, their certification in the country of origin and compliance with quality standards“.
The vaccine produced by the Serum Institute already has been approved by twelve health authorities from different countries, in addition to India.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos