[ad_1]

In this age group, vaccination is intended for people with a risk factor and a medical indication.
Misiones government sources denied that the province was vaccinating against the coronavirus unchangedCovid-19 people without co-morbidities who make up the age group among the 18 and 30 years old, as has happened in recent hours, especially in Puerto Iguazú.
coronavirus on mission
Health authorities confirmed to Misiones Online that the so-called “free vaccination “ reached the over 30 years old, as announced on Tuesday. Qwho are between 18 and 30 years old They can only receive the first dose against the coronavirus without having a risk factor and medical indication. Remember that none of the vaccines applied by Argentina has been approved for minors.
More vaccines
The national government is preparing a complex logistics operation that will include ten Aerolineas Argentinas flights to China to deliver 8 million Sinopharm vaccines in July. coronaviruss, official sources reported.
The first flight could leave this Friday around noon and, by the middle of next week, nine more would take off at the rate of one per day.added the sources.
A few days ago, the Minister of Health, Carla Vizzotti, announced the signing of a contract for the acquisition of 24 million Sinopharm vaccines, with a delivery scheduled between July and September and at a rate of eight million doses in each of the three months.
What will the vaccine research operations look like?
According to data collected by Telam on the operation, the first flight would take off from Ezeiza international airport this Friday, around 1:00 p.m., to arrive in Beijing, around midnight in China, after a technical refueling stop in Barajas. airport, in Madrid.000
Each flight will carry around 800,000 doses of vaccine, taking into account that there are 8 million doses divided into 10 flights and that all flights would maintain the same route and, in principle, the same schedules.
The use of Sinopharm in the country
So far, six million vaccines produced in China have arrived in the country, of which 5,195,200 have been carried on 7 Aerolineas Argentinas flights, while the rest have arrived on scheduled flights from Lufthansa, Qatar and KLM.
Sinopharm is one of three vaccines developed by Chinese laboratories, manufactured by the Chinese National Pharmaceutical Group Corporation (known as Sinopharm), and requires two doses and can be transported and stored at 2-8 ° C.
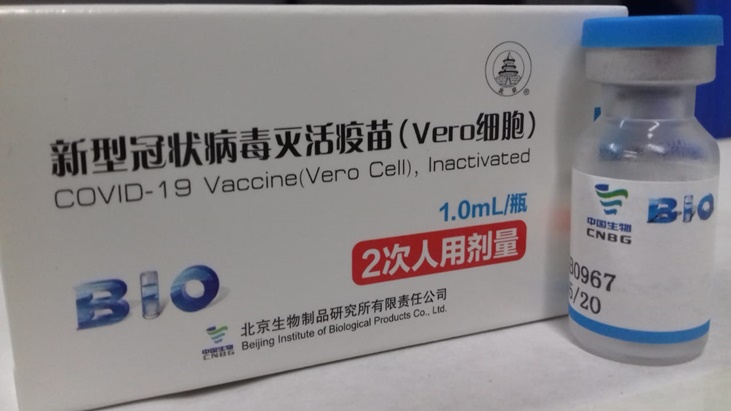
According to studies conducted by Chinese technicians, it has an efficiency of 79.34%.
It has been authorized by the Nation’s Ministry of Health for emergency use and the first shipment of this vaccine arrived at Ezeiza International Airport on an Aerolineas Argentinas flight which brought 904,000 doses to its warehouses. .
Initially, the vaccine was used as dose 1 for teachers, health workers, and people between 18 and 59 years of age with risky conditions, because the National Administration for Drugs, Food and Health Technologies (ANMAT) had recommended the use of the vaccine only in less than 60 years.
However, on March 25, Vizzotti announced that the ANMAT recommended that the ministry allow the emergency use of the Sinopharm vaccine in the over 60 age group and in this way the vaccine began to be used for advance the vaccination of this at-risk group.
The Sinopharm vaccine was developed in collaboration with the Beijing Institute of Biologics and is an inactivated vaccine, which means that it carries a version of the virus that is genetically engineered to be unable to reproduce, but that generates a immune response in the body. .
On December 30, Sinopharm, a Chinese state-owned pharmaceutical company, announced in a statement that the first interim analysis of the results of the phase III clinical trial showed that the vaccine achieves an efficacy of 79.34% after administration of 2 doses.
This report indicated that the vaccine is safe and that participants who received two doses produced a high level of antibodies against the virus, at a rate of 99.52%.
Sinopharm had already completed its clinical trials in the United Arab Emirates (UAE), where interim results gave it an efficacy of 86%.
The vaccine has been approved in 36 countries, including Argentina, including United Arab Emirates, Bahrain, Egypt, Jordan, Cambodia, Iraq, Morocco, Serbia, Pakistan, Seychelles and Hungary .
In Latin America, the government of Peru announced that it had signed a purchase agreement and letter of commitment with Sinopharm to access 30 million doses.
Other Chinese laboratories are also developing CoronaVac vaccines, from Sinovac Biotech – a vaccine currently applied both in Uruguay and Chile – and the vaccine called Ad5-nCoV or Convidecia, from Cansino Biologics with the Beijing Institute of Biotechnology . trials are also being carried out in Argentina by the Fundación Host.
LD-EP
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos