[ad_1]
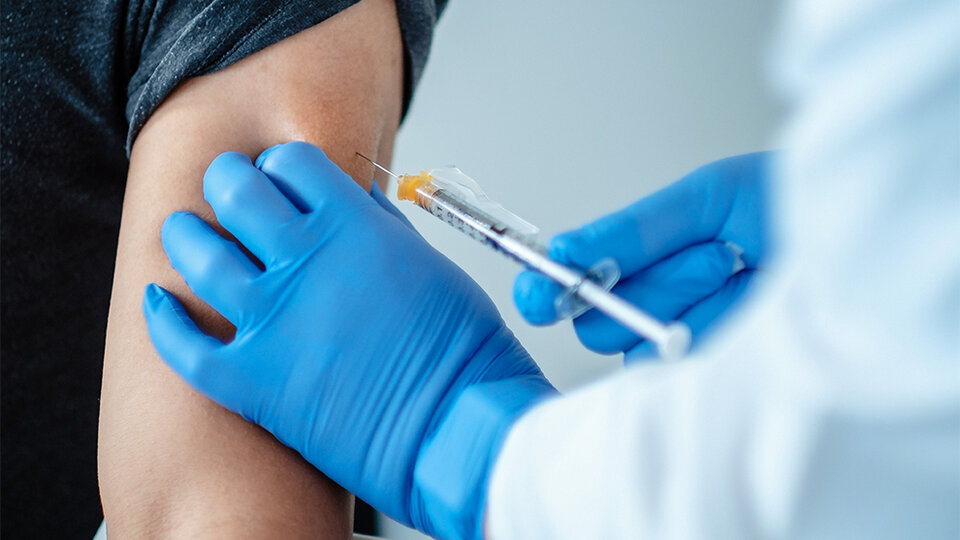
The President of the European Commission, Úrsula von der Leyen, assured in a brief appearance that this license opens “an important chapter in the fight against the pandemic” and will allow the launch of vaccination campaigns next Sunday “at the same time and in the same conditions “in all European countries, where the first doses will arrive from the Pfizer plant in Belgium” in the coming days “.
“It’s a good way to end this difficult year and finally start to turn the page on covid-19”, he added, calling the vaccine “a real product of European innovation”. that it was developed with the German partner BioNTech. , a victory he considered “a real achievement for the European Union”.
For his part, the director of the EMA, Emer Cooke, described the endorsement of the drug as “historic scientific achievement” and “a significant step in our fight against this pandemic which causes suffering and hardship to so many people in the world”.
This conditional license includes “the corresponding guarantees, controls and obligations” for pharmaceutical companies, and provides a “solid scientific basis for launching vaccination programs with an ongoing vaccine monitoring and surveillance plan,” Cooke said.
The President of the European Council, Charles Michel, saw this as “a decisive moment in the fight against covid-19” and called for the vaccination of the 446 million Europeans “in a safe, effective and equitable way” because vaccines are “Our ticket” to get out of the pandemic, regain our lives and explode our economies ”.
The new strain
One of the main concerns is that the latest mutation undergone by SARS-CoV-2, resulting in a new strain detected in the UK which accelerates transmission of the virus by up to 70%, is having direct effects on the efficacy of this vaccine. from Pfizer, or the one manufactured by Moderna that is expected to be approved by the EMA on January 6.
“At the moment, we can say that there is no evidence to suggest that the vaccine would not work against the new variant of the coronavirus. But we all have to do our part to prevent the spread of the disease: follow the advice of your health authorities, wear a mask, wash your hands and keep your distance, ”Cooke urged.
For the EMA, the new strain “is a question that must be scientifically evaluated anyway”, and recalled that vaccines “alone will not be the miracle solution that allows us to resume a normal life, but this authorization is definitely a big step in the right direction and an indication that 2021 may be better than 2020 ”, he celebrated.
The authorization issued to Pfizer is “conditional”, which means that the pharmaceutical company must keep the EMA informed “monthly” of the results of its monitoring of vaccination campaigns, which will allow the vaccine to be evaluated until the manufacturer has the guarantee of an official marketing authorization.
In an interview with Efe and other international agencies, Health Commissioner Stella Kyriakides warned that “it will only be possible to return to normal when there are a sufficient number of vaccinated citizens” because “the authorization of a vaccine, however effective I mean, it is not a quick fix.”
Side effects
The clinical trial of 42,000 people over the age of 16 who were injected with the Pfizer vaccine found that it was 95% effective, also in those over 65 and at risk groups, and was not not shown serious side effects, more beyond those common to other vaccines, such as fatigue, headache, muscle or joint pain, fever, chills and discomfort at the injection site.
Harald Enzmann, head of the CHMP, reiterated that “there was no evidence of unacceptable risks” from the vaccine, although he noted that there are “very little data on the use of the vaccine in women pregnant because there were not enough cases of such cases in the clinical trial, so it is recommended to go on a case-by-case basis, assessing the individual risk of each person ”.
In addition, as allergic reactions have been observed in countries where the vaccine has already been administered to citizens, such as the United Kingdom and the United States, the EMA asks to “monitor for 15 minutes after administration of injection ”vaccinated people.
He acknowledged that “the speed with which this vaccine has been developed and approved is a matter of concern to many Europeans”, which the EMA will try to combat “with transparency”, by publishing the information available to gain “confidence of citizens in the authorization ”which was granted to Pfizer.
“I want to assure you that the data we analyzed in our assessment meets the quality standards that we set for any vaccine. In fact, the main trial for this vaccine is one of the largest trials we have ever evaluated for a vaccine, ”added Enzmann.
EFE Agency
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos