[ad_1]
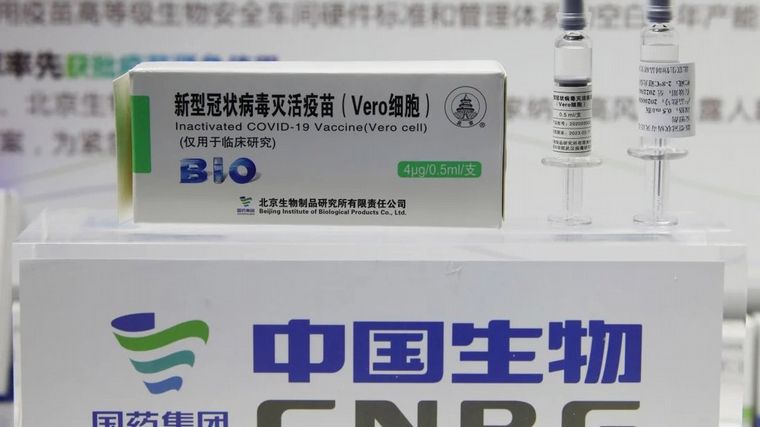
The million doses of Sinopharm immunize 500,000 peopleSince this drug, like most Covid-19 vaccines, requires two applications.
It is expected that in the first days of the week, the National Administration of Drugs, Food and Medical Technology (ANMAT) will issue the authorization to use the Sinopharm vaccine, the scientific name of which is BBIBP-CorV, in emergency conditions.
/ Embedded code reception /
Also watch
Fight against the coronavirus
/ End of embed code /
This approval method is provided for under the provisions of Annex I, point 5, of ANMAT 705/05, which authorizes the registration and use of vaccines in this type of situation.
In the case of the Sputnik V vaccine, on Saturday evening, presidential adviser Cecilia Nicolini left for Moscow, which will analyze in the coming days with the authorities of the Russian Direct Investment Fund (RDIF) the setbacks that have occurred in recent weeks and that affected the supply of doses to Argentina.
/ Embedded code reception /
/ End of embed code /
The new Minister of Health, Carla Vizzotti, confirmed this Sunday in statements on the radio that Chinese Sinopharm’s emergency use approval is “last resort”, since the ANMAT has received “information from the phase 3 trial, for interim analysis, as for all vaccines”.
Further, the official explained that the testing body “made specific requests and they were responded to,” a process which in Sinopharm’s case was favored because the Chinese state laboratory conducted simultaneously phase 3 studies in various regions of the world (United States of the United Arab Emirates, Egypt, Bahrain, Serbia, Peru, Pakistan, Morocco) but also in Argentina.
/ Embedded code reception /
Also watch
Fight against the coronavirus
/ End of embed code /
In fact, in the country the trials were carried out on a control population of 3008 volunteers who participated in a “randomized study” (they receive a random vaccine or a placebo) of the association of the Chinese pharmaceutical group with the Vacunar network and the Host Foundation plus the ELEA laboratory (from Grupo Insud) as a local sponsor.
The head of the Sinopharm trials in Argentina was infectious disease specialist Pedro Cahn, founder of the Fundación Huiuda.
/ Embedded code reception /
/ End of embed code /
“It’s extremely safe, in the trials we didn’t see any serious vaccine-related adverse events, so from a safety point of view I can give you a guarantee,” Cahn said ahead of the Telam consultation. , adding: “From a safety point of view In terms of efficacy, they (due to the laboratory that produces it) have reported efficacy similar to that of other vaccines.”
It is good news that these vaccines are coming, as they will allow us to expand the menu of offers and speed up the vaccination process.
On the other hand, and beyond the immediate, the arrival of this million doses of Sinopharm could be the first batch of a series of new purchases, sources which follow in detail the negotiation assured Telam.
In fact, the pharmaceutical group that developed the drug, the China National Pharmaceutical Group, has already warned that from March it will be ready to periodically supply significant quantities, well over a million.
As he could know Telam, the commercial contract for the acquisition of the million doses was concluded between Wednesday and Thursday. It was one of the last decisions of Ginés González García as head of the Ministry of Health.
/ Embedded code reception /
Also watch
Government scandal
/ End of embed code /
In dialogue with Radio 10, Vizzotti confirmed this morning that in the context of discussions with the “Sinopharm laboratory, China”, referring to the CNPG group, “the agreement for one million doses has been signed”.
The deal was made, among other things, after the Beijing-based pharmaceutical company agreed to lower the price of each unit from $ 30 to $ 20, as the head of state himself announced he two weeks ago.
“We offered to do an initial contract and I want to clarify that they have improved the price for us, and we are at $ 20 each dose“, he said two weeks ago in the newspaper interview Page 12.
/ Embedded code reception /
Also watch
Scandal in Buenos Aires
/ End of embed code /
Another special feature of this vaccine is that it is based on the inactivated Covid-19 virus (the dead version of the germ that causes the disease) and does not use adenoviral vectors (a virus which, since it does not have the gene that reproduces it can carry the genetic material of another virus).
In addition, the development of Sinopharm has a peculiarity that can be considered a logistical advantage: it does not require refrigeration below 0 degrees Celsius and it can be maintained at temperatures between 2 and 8 degrees, than a common refrigerator can provide.
Argentina’s Ambassador to China, Sabino Vaca Narvaja, and Sinopharm chief Liu Jinzhen discussed all of these details, as well as operational issues related to the plane transfer from Beijing to Ezeiza airport.
/ Embedded code reception /
Also watch
Government scandal
/ End of embed code /
On the other hand, with regard to the supply of doses of Sputnik V by the Russian Federation, the government hopes that Nicolini’s visit to Moscow will establish a delivery schedule that will be maintained with some predictability over time.
At this point, the problem that has arisen in recent weeks is related, on the one hand, to the expansion of demand received by the Russian Direct Investment Fund.
Countries that had not initially expressed interest in purchasing the vaccine of Russian origin or that were waiting to receive doses from other international suppliers are now contacting Moscow.
/ Embedded code reception /
Also watch
Government scandal
/ End of embed code /
The other drawback is linked to the technology transfer that the Gamaleya Institute has carried out on laboratories in third countries which have a tradition in the field of pharmaceutical research, such as India.
In the case of Argentina, in the first tranche of the negotiation, it was agreed that after the first supplies of vaccines (delivered directly to Moscow), the following lots would be supplied by these associated laboratories, located outside Russia.
As far as is known, there have been delays in Gamaleya’s second quality check on vaccine production in these locations and this has changed plans.
/ Embedded code reception /
/ End of embed code /
On his third trip to Moscow – the first two were made with Vizzott – Nicolini will be on a mission to dispel doubts and clearly foresee what will happen.
“Nicolini traveled to the Russian Federation to be able to follow closely and closely the progress of technology transfer, production and adherence to schedule,” Vizzotti said yesterday of the Sputnik vaccine.
“The idea is to set up a schedule for the next deliveries,” he noted.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos