[ad_1]
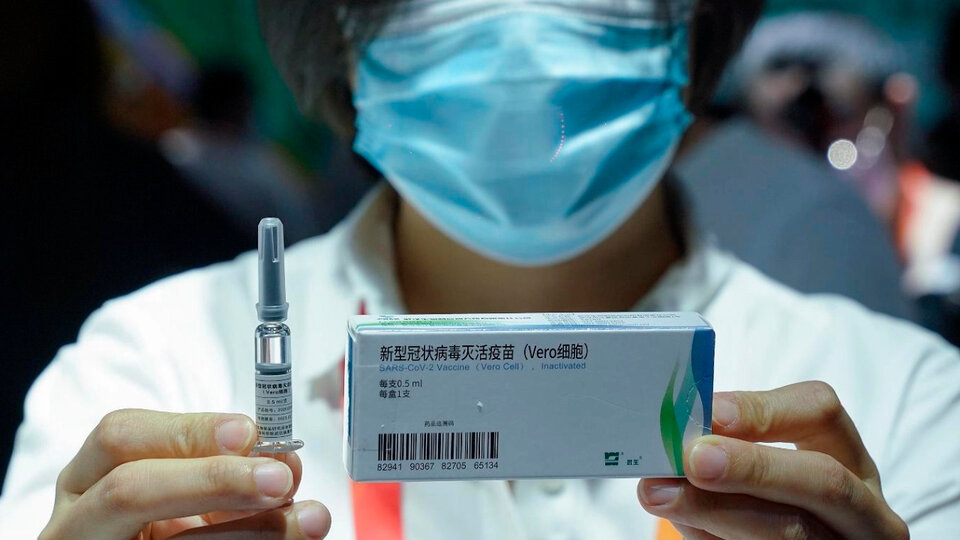
Chinese laboratory Sinopharm announced that one of its covid-19 vaccines has 79% efficiency and officially sent a application for approval to the Beijing administration so that the health authorities give the green light to marketing in the country where the pandemic broke out.
While the percentage of efficiency is lower initially that the Gamaleya Institute for Sputnik V (91.4 percent), Pfizer / BioNTech (95) and Modern (94.1), it is not excluded that subsequent studies report improvements at this stage. English Astrazeneca, associated with the University of Oxford, said for his part that his vaccine is 70% effective but could reach 100% with two doses.
Anyway, Chinese laboratory has two vaccines in development as well as one produced with the Institute of Biologics in Wuhan, the city where the first coronavirus epidemic was detected and is the first Chinese pharmaceutical group to report data on the effectiveness of a vaccine in preparation.
Sinopharm has carried out tests in various Latin American countries, including Argentina and Peru. This is a so-called “inactivated” vaccine, which means that it uses a very classic method that uses a “killed” virus to trigger an immune reaction in the person.
“Protective effect of vaccine against covid-19 is 79.34 percent”said the Beijing Biological Products Institute, a subsidiary of Sinopharm. On its website, the entity indicated that the results of phase 3 Tests show that the safety levels are “good” and that all participants developed high levels of antibodies after receiving both doses.
The peculiarity of the case is that the authorities of the United Arab Emirates (UAE), one of the countries which participated in the tests, had indicated at the beginning of the month that this product had 86% efficiency.
Currently, four vaccines developed in China have reached phase 3 clinical trials: by Sinopharm and one of Sinovac are based on inactivated versions of the infectious agent, while those caused by CanSino organic products uses an adenovirus vector to produce specific antibodies against SARS-CoV-2.
While the request for approval has been submitted to the Chinese government, the authorities have not yet authorized the marketing of any vaccine. They did this to be used in an “urgent” need.
In fact, at least one million health workers, employed in jobs deemed to be at risk, and students and diplomats who must travel abroad have already experimental vaccine.
Chinese health authorities have reiterated in recent months that no serious side effects detected during trials with the various vaccine candidates developed in the country.
A little over a month ago, Sinopharm Chairman Liu Jingzhen said that nearly a million people in China had already received one of these “emergency” vaccines and that only a small number had experienced mild side effects.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos