[ad_1]
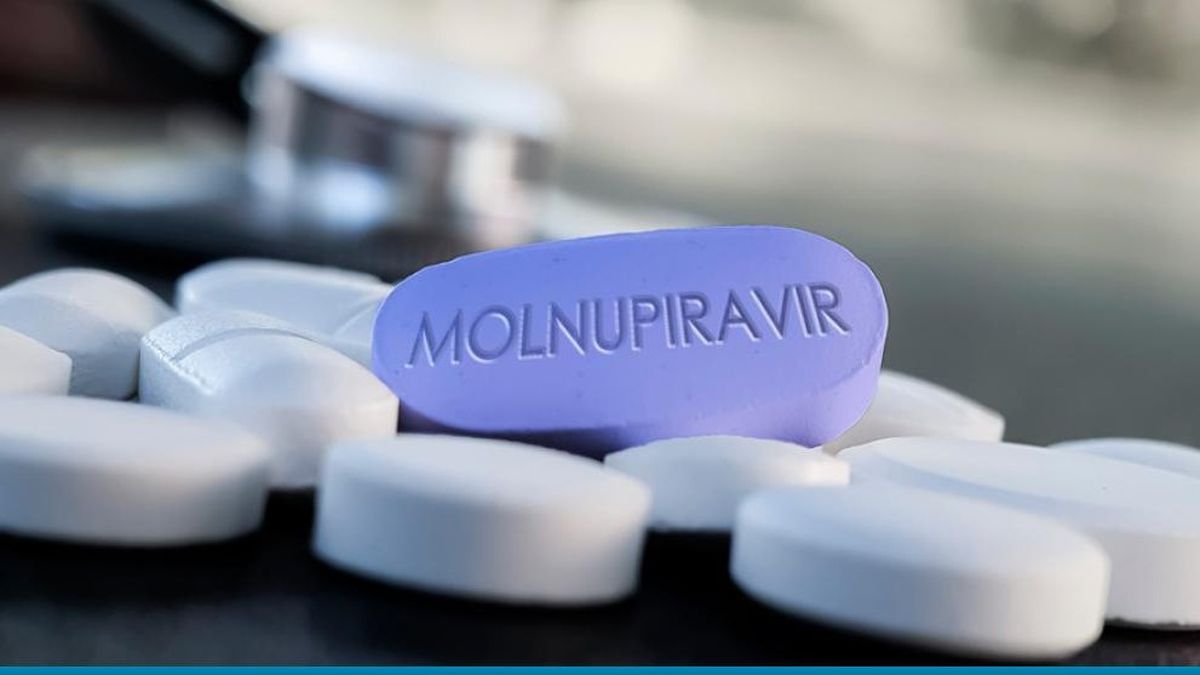
According to Merck and Ridgeback, the pill “reduced the risk of hospitalization or death by about 50% compared to placebo for patients with Covid-19 mild or moderate in the positive interim analysis of the phase 3 study ”.
According to the Merck press release, molnupiravir was administered to patients who had already contracted the virus, showing its effectiveness in reducing severe cases but also in treating the viral variants Gamma, Delta and Mu, which accounted for nearly 80%. of cases evaluated.
“With these compelling results, we are optimistic that molnupiravir could become an important drug in the global pandemic effort,” said Robert M. Davis, CEO and President of Merck.
The pill is awaiting clearance from the US Food and Drug Administration (FDA) and will be sent to other regulatory agencies for approval.
With this drug, the company has made a breakthrough in the treatment of coronavirus, since it is an easier pill to administer.
According to the document, the MOVe-OUT trial was a global, phase 3, randomized, placebo-controlled, double-blind study in adult outpatients with Covid-19 mild to moderate laboratory confirmed, with at least one risk factor and associated symptoms.
The primary objective was to assess the action of molnupiravir, compared to placebo, based on the evaluation of the percentage of participants hospitalized and / or deceased from the time of its administration until day 29.
Although these are preliminary results, infectious disease doctor Eduardo López told the agency that the treatment “shows promise” although he said it “cannot be used in any way and that the final results of phase 3 should be awaited “.
According to the hospital specialist Gutiérrez, “this is the generation of antivirals that directly target the virus; In other words, it is the line of research that attacks the virus. It does not prevent disease like the vaccine, with varying degrees of effectiveness ”.
“If approved, the disease could be treated at an early stage and if it had been before, complications in serious developments or death would have been avoided,” said the doctor, who clarified that he is currently being analyzed to see if it can “eliminate the virus”. respiratory tract. “
For his part, infectologist Luis Camera estimated that these oral treatments to treat the virus “are very important because it gives the feeling that the Covid19 will be there for a long time”.
“Although these are preliminary results, if it is confirmed that they reduce the rate of hospitalization and death to 50%, if they had had it before, there would not have been the restrictions we had or quarantines, ”Camera said.
According to the doctor, “antiviral treatments have been tried and all have failed” and confirmed that there is another study in Argentina, antiviral, of an antiparasitic which is being investigated.
Being able to have antiviral supplementation as a treatment for the coronavirus “can be useful for hundreds of patients,” he said.
“If an epidemic occurs with the Delta variant, in addition to the two vaccines, it could also have a window of action for anyone who becomes infected, vaccinated or not,” he explained in dialogue with Telam.
Ridgeback Biotherapeutics CEO Wendy Holman said that because the virus is circulating widely and the available treatment options require access to a health facility, “antiviral treatments that can be taken at home to keep people with the disease of Covid-19 outside the hospital, they are extremely necessary ”.
“Molnupiravir is an experimental orally administered form that inhibits the replication of SARS-CoV-2, in the preclinical stages and has been shown to be effective for the prophylaxis, treatment and prevention of transmission,” detailed the official statement. of the laboratory.
In a statement posted on Twitter, the company said “more tools and treatments are urgently needed to tackle the pandemic of Covid-19, which has become one of the leading causes of death and continues to deeply affect patients ”.
In addition, he explained that molnupiravir “is an experimental orally administered form of a potent ribonucleoside analogue that inhibits the replication of SARS-CoV-2, the causative agent of Covid-19”.
He added that “molnupiravir has been shown to be active in various preclinical models of SARS-CoV-2, including for prophylaxis, treatment and prevention of transmission.”
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos