[ad_1]
Health authorities of UK warned that people with a “significant” history of allergies should not receive the coronavirus vaccine from Pfizer / BioNTech, after two health workers reported reactions on Tuesday, November 8 after receiving the injection, although both have already recovered.
Stephen powis, medical director of the NHS (the UK’s public health service), said the two workers, who had a history of allergic reactions, were recovering well.
The Independent Medical and Health Regulatory Agency (MHRA) recommended that “people with a significant history of allergic reactions” not receive the injection. “Significant” reactions include responses to drugs, vaccines, or food.
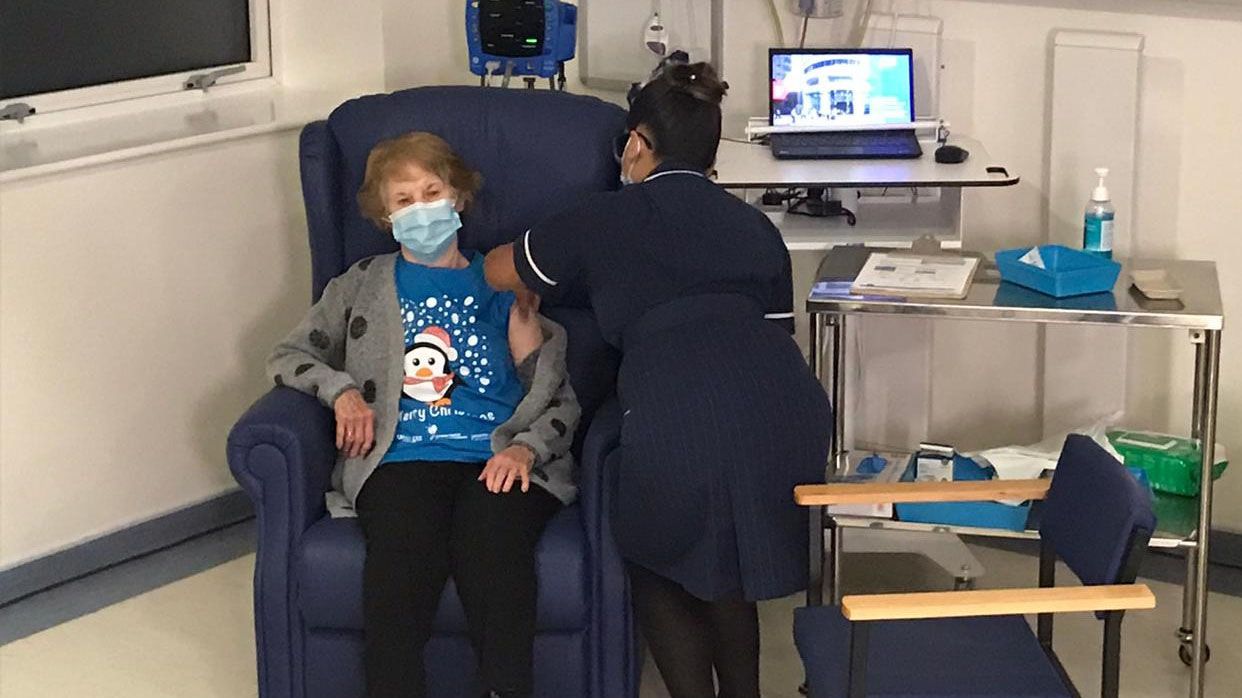
The alert comes after the UK began its largest vaccination campaign since 1948 this week, becoming one of the first countries to apply an approved COVID-19 vaccine. During the so-called V-Day (Vaccine Day, but also refers to Victory Day, in reference to World War II), the first to receive the injection was a 90-year-old woman named Margaret keenan, while the second patient to receive the vaccine was William Shakespeare, an 81-year-old man from the town of Warwichshire.
Health and social assistance workers are the first, along with the elderly (the population most at risk of the pandemic), to receive the injection, which is given in two doses, 21 days apart. The British have received 800,000 doses of an order of 40 million, and hope to accumulate 8 million by the end of December, according to the agency. AFP.
90-year-old woman first to be vaccinated against coronavirus in UK
Albert BourlaThe CEO of Pfizer acknowledged on Tuesday that he understood global concerns about the speed of production and approval of the vaccine, but insisted no shortcuts were being taken. The coronavirus vaccine has been tested “in exactly the same way that any other vaccine in circulation is tested,” he told a press conference in Geneva.
The company added that the MHRA has reported allergic reactions, but said the injection was “generally well received without any serious safety concerns” in Phase 3 trials, which involved more than 40,000 people.
FF
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos