[ad_1]
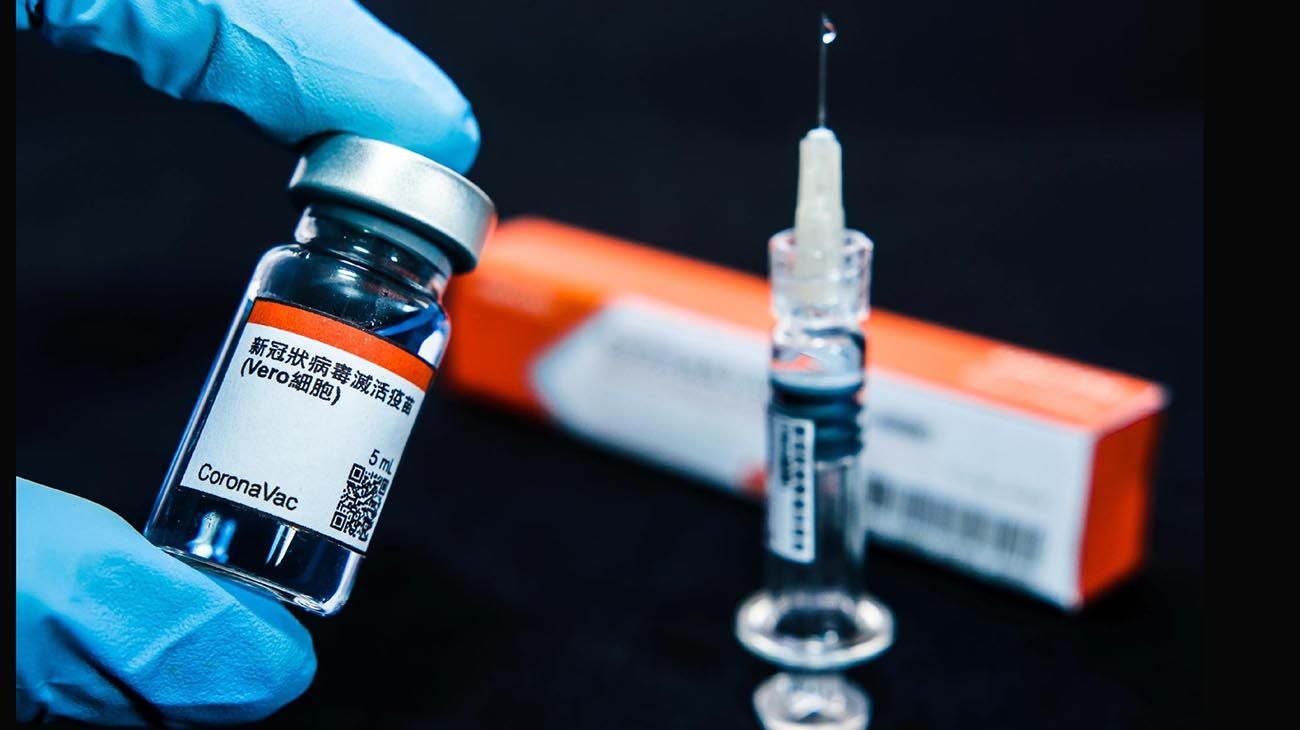
The Brazilian institute Butantan assured this Wednesday that the tests of Coronavac vaccine, which she is developing with a Chinese lab, was successful and she hopes to start applying the coronavirus immunizer in January.
In addition, the president of Butantan, Dimas Covas, indicated that he had exclusivity for the distribution of the vaccine in all Latin American countries except Chile, and that could sign a contract with the Argentine government this week.
CoronaVac was created by the Chinese private laboratory Sinovac Life Science and achieved the required “superiority in efficacy” (50%) against the coronavirus required by the World Health Organization (WHO) in trials with 13,000 volunteers from Brazil, the institute said.
“The data corroborate that this is the safest vaccine on the market,” announced the director of the Butantan Institute, Dimas Covas.
How will the operation of distribution and application of the Russian vaccine throughout the country be
“We have reached the level of efficiency that allows us to apply for emergency vaccine use registration here and in China. It’s a historic day for Brazilian science because of the hope it brings to Brazilians“said Covas, who detailed the dispatch to regulator Anvisa will take place after Sinovac Life Science analyzes the results sent to the Chinese laboratory on Wednesday within a period of no more than 15 days.
In mid-November, it became known that the Sinovac vaccine was safe and tolerable and could induce a rapid immune response against the coronavirus, based on the results of early and intermediate clinical trials, which were published in the medical journal Infectious Diseases The Lancet.
Argentina to negotiate to buy Chinese Coronavac vaccine from Brazil
The level of antibodies induced by the vaccine was lower than in people who have recovered from the disease, while it was able to protect those inoculated from infections caused by the virus, according to the source.
CoronaVac was developed by Chinese biopharmaceutical manufacturer Sinovac Biotech. The formula has undergone Phase I and II randomized, double-blind, placebo-controlled clinical trials involving more than 700 healthy adults between the ages of 18 and 59, according to The Lancet.
The vaccination is carried out with two doses with an interval of 14 days and the experimental vaccine is effective, which makes it fit for emergency use amid the pandemic, said Zhu Fengcai, one of the authors of the findings. Currently, the candidate vaccine is in phase III clinical trials to confirm its effectiveness.
ds
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos