[ad_1]
After AstraZeneca’s vaccine begins to be linked to an unusual blood clot condition, Johnson & Johnson, based on the same technology, are the targets of the same suspicions.
Yesterday, the Pan American Health Organization (PAHO) recommended continuing to apply the AstraZeneca anticovid vaccines, being examined for their possible link with exceptional cases of blood clots and permanently suspended in Denmark for “rare side effects” “But” serious “.
“Almost 200 million people worldwide have received the AstraZeneca covid-19 vaccine and reports of adverse reactions are very rare,” PAHO director Carissa Etienne said at a press conference.
Here’s what we know so far:

What has been observed?
In both cases, suspicion arose after the detection of several cases of thrombosis in vaccinated persons. These are not simple thromboses, like phlebitis, but very unusual conditions. First, because of their location: they affect the veins of the brain and to a lesser extent the abdomen, said the European Medicines Agency (EMA) on April 7 of AstraZeneca.
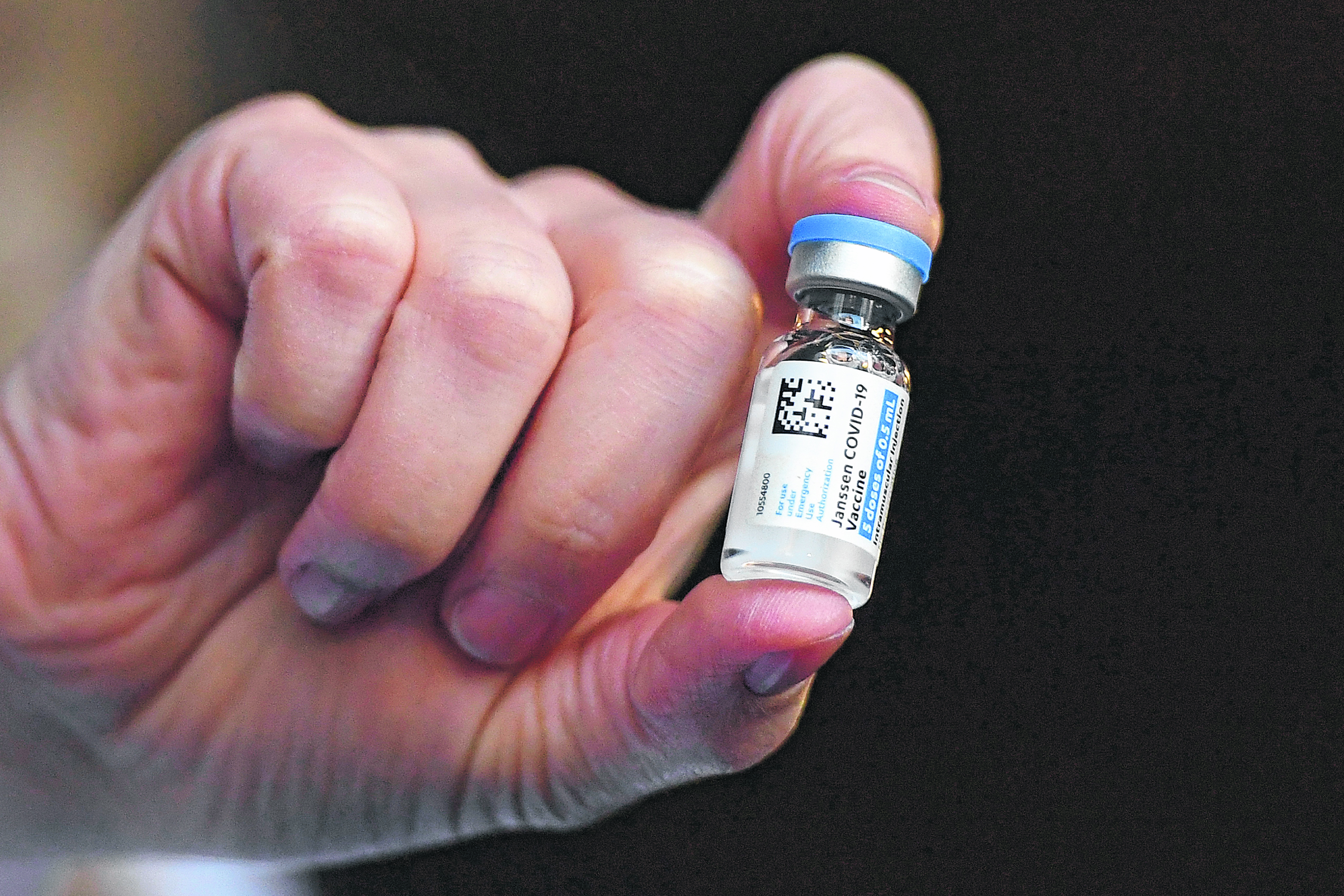
The Johnson & Johnson vaccine also showed “cerebral venous sinus thrombosis,” US health officials, the FDA and the CDC said on Tuesday.
These conditions occur with a drop in the level of blood platelets, so that at the same time as the blood clots the patient may suffer from bleeding.
The EMA first acknowledged on April 7 that these problems could be caused by the AstraZeneca vaccine.
However, this link has not yet been formally established with the Johnson & Johnson vaccine, approved but not yet administered in Europe under the name Janssen.
In the United States, his post was suspended Tuesday pending a scientific response.
And what is it due to?
These problems could be related to the technique of the two vaccines “viral vectors”. This is based on taking another virus as a carrier, which is modified so that it carries genetic information in the body that is capable of fighting COVID.
Both use an adenovirus, a very common type of virus. AstraZeneca is a chimpanzee adenovirus and Johnson & Johnson is a human adenovirus.
“Everything indicates that it is due to the adenovirus vector”, explained on Twitter Mathieu Molimard, French specialist in pharmacology, recalling that this type of problem does not occur with the Pfizer / BioNTech and Moderna vaccines, which use the technique of messenger RNA.
For now, it is not known whether these pathologies are also recorded with another vaccine using an adenovirus, the Russian Sputnik. This is allowed in about 60 countries but not in the European Union or the United States.
What are the mechanisms?
There are several indications of an excessive immune reaction caused by these vaccines.
In a study published online on March 28, German and Austrian researchers compared with another known mechanism.
The phenomenon “clinically resembles heparin-induced thrombocytopenia (HIT),” said the team of scientists led by Andreas Greinacher of the University of Greifswald.
HIT is an unusual, severe, and abnormal immune reaction triggered in some patients by the blood thinner heparin.
That’s a “plausible explanation,” the EMA assessed on April 7, calling for further studies.
What’s the risk?
This is the main question.
According to figures from the EMA, as of April 4, 222 cases of atypical thrombosis had been detected after 34 million injections performed with AstraZeneca in the 30 countries of the European Economic Area (EU, Iceland, Norway, Liechtenstein) and in United Kingdom, with a balance of 18 dead until March 22.
The thromboses occurred “during the two weeks following the vaccination”, according to the EMA.
In the case of Johnson & Johnson, US authorities recorded six cases, including one death, among more than 6.8 million doses administered, and symptoms appeared between 6 and 13 days after the injection.
But, as with all medications, the key is to weigh the risks against the benefits.
“The vaccine’s overall benefits in preventing COVID-19 outweigh the risks of side effects,” the EMA said of AstraZeneca.
What are the risk factors?
Currently, the majority of AstraZeneca cases have occurred in “women under the age of 60,” according to the EMA. The six cases detected in the United States with Johnson & Johnson were women between the ages of 18 and 48.
But it is too early to draw any conclusions. So far, “we haven’t identified any specific risk factors,” said AstraZeneca’s EMA.
: 30 years in the United Kingdom, 55 years in France, Belgium and Canada; 60 in Germany and the Netherlands and 65 in Sweden and Finland.
“We don’t have just one vaccine, we have several. For this reason, it seems to me that it makes sense to reserve AstraZeneca for the elderly, ”Sandra Ciesek, virologist at Goethe University in Frankfurt, told Science magazine.
UK officials have released a chart showing covid-19 poses a six times higher health risk than the vaccine in 20-29 year olds.
But in the 60-69 age group, the risk is 600 times greater.
SIGN UP TO STAY AT HOME
Every day at 7 p.m. to accompany the end of the day.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos