:quality(85)//cloudfront-us-east-1.images.arcpublishing.com/infobae/MDYTR5AXV6454PKOVF672OO4JA.jpg)
[ad_1]
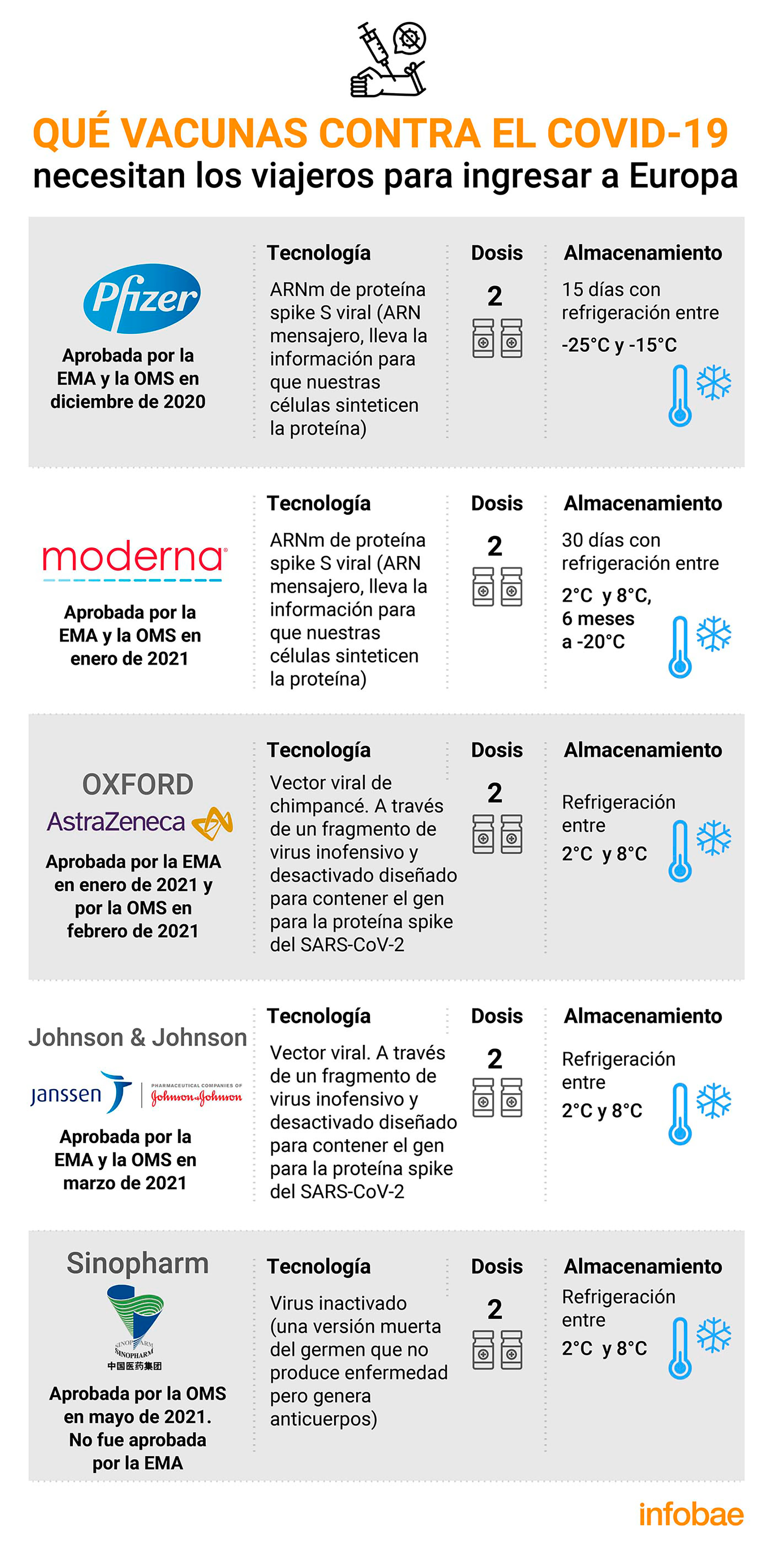
The European Union has reached an agreement to reopen its borders to tourists vaccinated against COVID-19 in the coming weeks. The condition is that they have completed the vaccination cycle, with two doses in almost all existing vaccines, at least fourteen days before their trip to Europe. But it can’t be just any vaccineThose traveling to countries in the bloc must be immunized with one of the developments authorized by the European Medicines Agency (EMA) or by the World Health Organization.
Yes indeed, the list boils down to Pfizer, Moderna, AstraZeneca, Johnson & Johnson and Sinopharm. Of course, as the European authorities have confirmed, the list will be expanded as the EMA and WHO authorize more vaccines.
Pfizer
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/HMY7ZH6UU2P7GQODZ5UVGZVDPU.jpg 420w)
The European Union approved the Pfizer / BioNTech vaccine in December against the coronavirus, which opened the door to the first vaccinations in the 27 member countries after the holidays. The EMA cleared the product for commercialization and, hours later, the EU’s executive arm, the Commission, gave the final green light. Almost in parallel, he received the green light from the WHO.
The vaccine developed by the American giant Pfizer and the German company BioNTech had shown, when approved, an efficacy of 95% in international clinical trials in which two doses were administered three weeks apart.
The regulator has performed a “continuous review” of laboratory and clinical trial data as it arrives. Normally, the agency does not review the data until it has been fully collected.
The formula is based on messenger RNA technology, which contains DNA instructions that allow cells in the human body to generate certain protective proteins. An efficacy of 95% against the SARS-CoV-2 virus was obtained seven days after the second dose of vaccine and 28 days after the first.
Modern
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/3Z4O6HEV6GLS7DT64YTKZMI5CU.jpg 420w)
The EMA and WHO approved Moderna’s vaccine in January 2021. This vaccine, based on messenger RNA technology, was developed by a pioneering startup in anticovid vaccines, it has characteristics similar to those developed by Pfizer-BioNTech, with an efficiency of 94.1%. Moderna’s formula, like Pfizer’s, needs to be super-frozen, but not so low, close to -20 degrees.
The vaccine is given as two injections into the arm, 28 days apart. The most common side effects of the vaccine were usually mild or moderate and got better a few days after vaccination. These include pain and swelling at the injection site, fatigue, chills, fever, swollen or tender lymph nodes under the arm, headache, muscle and joint pain, nausea, and vomiting.
Oxford-AstraZeneca
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/WOT32W6SFQALVBCS4W7G3SZK5A.jpg 420w)
The EMA gave the green light at the end of January for conditional use of the vaccine AstraZeneca and the University of Oxford in the European Union (EU) in January 2021 and the WHO followed suit in February this year.
This vaccine was developed by researchers at the University of Oxford in collaboration with the British pharmaceutical giant AstraZeneca. It is a “viral vector” vaccine: it is based on another virus (a chimpanzee adenovirus) that has been weakened and genetically modified to prevent the coronavirus from reproducing in the human body. The way it introduces genetic material into cells, ordering them to attack SARS-CoV-2, has been called a ‘Trojan horse’.
It has the advantage of being inexpensive (around 2.5 euros or 3 dollars per dose, with variations depending on local production costs). AstraZeneca agreed to sell it for no profit. Too it is easy to store: it can be stored at the temperature of a refrigerator, between 2 ° C and 8 ° Cunlike Moderna and Pfizer / BioNTech vaccines, which can only be stored long term at very low temperatures. This facilitates large-scale vaccination.
This drug is also distributed under the Covax program in developing countries.
Referring to the rare cases of thrombi in people immunized with this formula, the European Medicines Agency (EMA) has stated that “no cause and effect relationship has been proven, but it is possible”, and the benefits of the coronavirus immunizer always outweigh the risks.
Johnson & johnson
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/JFBPC5A2J7YXLXFOJIBVC2ZWMI.jpg 420w)
This vaccine was, in mid-March, the fourth to obtain authorization from the European Medicines Agency (EMA), after those of Pfizer-BioNTech, Moderna and AstraZeneca. WHO allowed it almost simultaneously.
The vaccine is supported by a viral vector, applying a technology already used by this company, in particular against the Ebola virus. In addition, it uses another attenuated virus as a carrier, transformed to add genetic instructions for part of the virus responsible for covid-19. Once it reaches the cells, it produces a protein typical of SARS-CoV-2 (virus from the same family that caused severe acute respiratory syndrome in Southeast Asia in 2002-2004), which enables the system immune system to recognize it. This is the first single dose to receive authorization from the World Health Organization (WHO).
For the European Medicines Agency (EMA), the Johnson & Johnson COVID-19 vaccine can be used as the benefits continue to outweigh the risks. The European regulator considers that the thrombi suffered by some vaccinated people will be considered as a “very rare” side effect of this vaccine.
“The EMA recognizes a possible link with the very rare cases of unusual thrombi associated with low blood platelets,” the agency said, noting that this “confirms that the overall benefit / risk balance remains positive.”
The chief financial officer of Johnson & Johnson (J&J) had previously assured that the laboratory had “complete confidence” in the vaccine and hopes to find “quickly” a solution with the regulators for its use.
This is an important decision, as several European countries have thThis single dose vaccine to speed up your vaccination campaign.
Sinopharm
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/XRND32OOME7JQNN2WJWM6FR72Y.jpg 420w)
Sinopharm is the first Chinese injectable to receive WHO approval and the sixth vaccine that he authorizes after having verified that it meets the required efficacy and harmlessness criteria.
This approval will allow the COVAX platform for equitable access to vaccines to start negotiations with the company Sinopharm to acquire its vaccines and distribute them among countries that do not have access to them.
The WHO approval came after it was reported that this vaccine provides 65% protection for people under the age of 60 to prevent serious illness and complications from coronavirus infection. The expert committee recommended the vaccine for people over 18 years of age.
Clinical trials with the Sinopharm vaccine have been conducted in China, United Arab Emirates, Bahrain, Jordan, Egypt, Peru and Argentina. In Argentina, it was cleared for emergency use on February 21 by the Nation’s Ministry of Health.
First, it was allowed for people under 60. But, at the end of March, specialists who are part of the National Commission of Immunization and the National Commission for the Safety of Vaccines argued that there was sufficient evidence of its effectiveness and safety when ‘it was also applied in people over 60 years old.
Regarding the efficacy of the vaccine, Preliminary results from Phase 3 indicated that it offers 79.34% protection starting 14 days after receiving both doses.
Sinopharm is not authorized by the European agency but since it has the green light from the WHO, people vaccinated with it will also be able to enter Europe.
What will happen to those who have been vaccinated with Sputnik V
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/NHYQZCVSM5C3B6LW3ECEGZHDLE.jpg 420w)
At the moment, the Russian development it has not been authorized by the EMA. Its experts completed the Good Clinical Practice Inspection with Russian Development on May 13 and are now visiting their factories, while monitoring vaccination campaigns to determine how long vaccines are protected and whether third doses are needed.
The reports of the two inspections will be part of the final dossier of the Russian vaccine study, an ongoing evaluation process that could lead to a conditional use license in the EU.
The inspection of factories where vaccines are produced in Russia aims to ensure that “adequate standards and controls for a careful manufacturing process” are met and to ensure “uniformity in the manufacture of the product”. So far, there is no precise date to know the conclusion of these investigations.
However, at WHO, the Russian inoculant is in the final phase of evaluation. If in the coming weeks, the world body decides to add it to the list, it would allow the millions of people vaccinated with the Russian vaccine – widely used in Argentina, for example – to travel to the European bloc as a tourists.
KEEP READING:
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos