[ad_1]
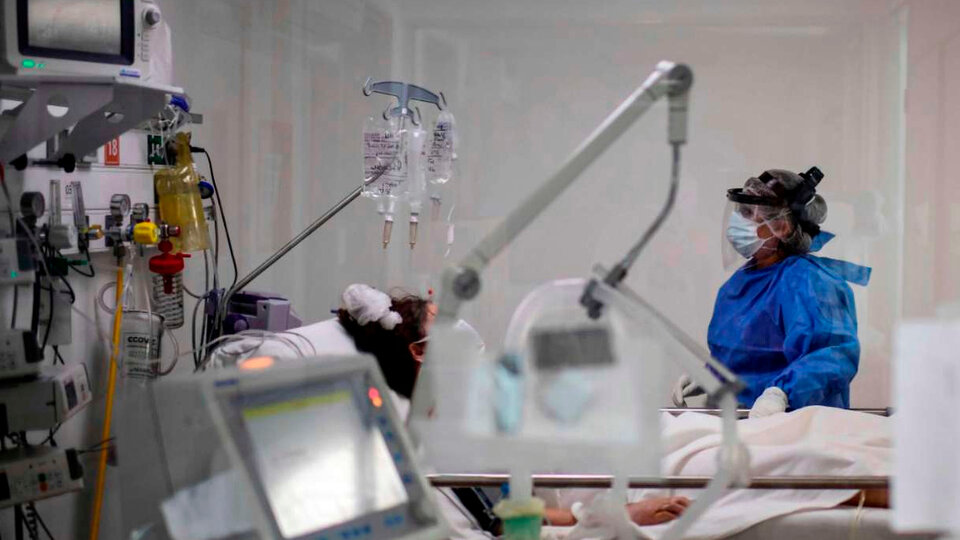
The World Health Organization (WHO) announced on Wednesday the start of trials with three possible new treatments for severe cases of covid. 600 hospitals in countries around the world are participating in the study, including Argentina.
Medicines will be used artesunate, used so far in cases of malaria; imatinib, which is used in certain types of cancer; and infliximab, prescribed for rheumatoid arthritis and diseases that attack the immune system.
The drugs were selected by a panel of independent experts, “given its potential for reducing the risk of death in patients with COVID-19,” said the Geneva-based organization.
Medications that didn’t work
With these drugs, the second phase of the Solidarity trials, which in its first phase did not obtain positive results after having tested four treatments in patients: hydroxychloroquine (initially created against malaria), the antiviral remdesivir, interferon, or the antiretrovirals lopinavir and ritonavir.
WHO concluded late last year that none of these four treatments made it possible to significantly reduce the length of hospital stays, deaths from COVID-19 or the number of people requiring ventilation.
Two effective treatments for severe cases of covid
Unlike the rapid development of covid vaccines, with more than a dozen already in use worldwide, WHO currently recognizes only two effective treatments for severe cases: dexamethasone, a corticosteroid, and the use of interleukin-6 antagonists.
While the former is readily available worldwide, due to the low price of dexamethasone, interleukin-6 is an expensive treatment which the WHO says would not be available to most patients in countries. in development.
The Solidarity trials (Solidarity PLUS in this new phase) will involve thousands of researchers in 600 hospitals in 52 countries, including Argentina.
What drugs to test
Produced by the Ipca laboratory, it is currently used to treat malaria. It will be given to COVID patients intravenously over 7 days, using the standard recommended dose for the treatment of severe malaria.
Artesunate is a derivative of artemisinin, an antimalarial drug extracted from the herb Artemisia annua. Artemisinin and its derivatives have been widely used in the treatment of malaria and other parasitic diseases for over 30 years.
Produced by Novartis, it is used to treat certain types of cancer. It will be administered orally, once a day, for 14 days. The dose used is the standard maintenance dose that patients with hematologic malignancies receive for long periods of time.
Preliminary clinical and experimental data suggest that imatinib reverses lung damage.
Produced by Johnson & Johnson, it is used to treat diseases of the immune system. It will be administered intravenously as a single dose. The dose used is the standard dose that patients with Crohn’s disease receive for long periods of time.
Infliximab is a TNF alpha inhibitor. Anti-TNF biologics have been approved for the treatment of certain inflammatory autoimmune conditions for over 20 years.
Where the new study will be done
WHO will carry out the survey in 600 hospitals around the world. Argentina will participate in the trial, although it has not yet been formalized which medical centers will be involved.
The participating countries are: Albania, Argentina, Bahamas, Bangladesh, Belize, Bolivia, Botswana, Brazil, Canada, Colombia, Dominican Republic, Ecuador, Egypt, Ethiopia, Finland, Georgia, Guyana, Honduras, India, Indonesia, Iran, Ireland, Saudi Arabia, Italy, Jamaica, Kenya, Kuwait, Latvia, Lebanon, Lithuania, Macedonia, Malaysia, Mali, Mexico, Mozambique, Niger, Nigeria, Norway, Oman, Pakistan, Panama, Paraguay, Peru, Philippines, Portugal, Romania, Sierra Leona, Spain, Switzerland, Trinidad and Tobago, Zimbabwe.
.
[ad_2]
Source link
 Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos
Naaju Breaking News, Live Updates, Latest Headlines, Viral News, Top Stories, Trending Topics, Videos