[ad_1]
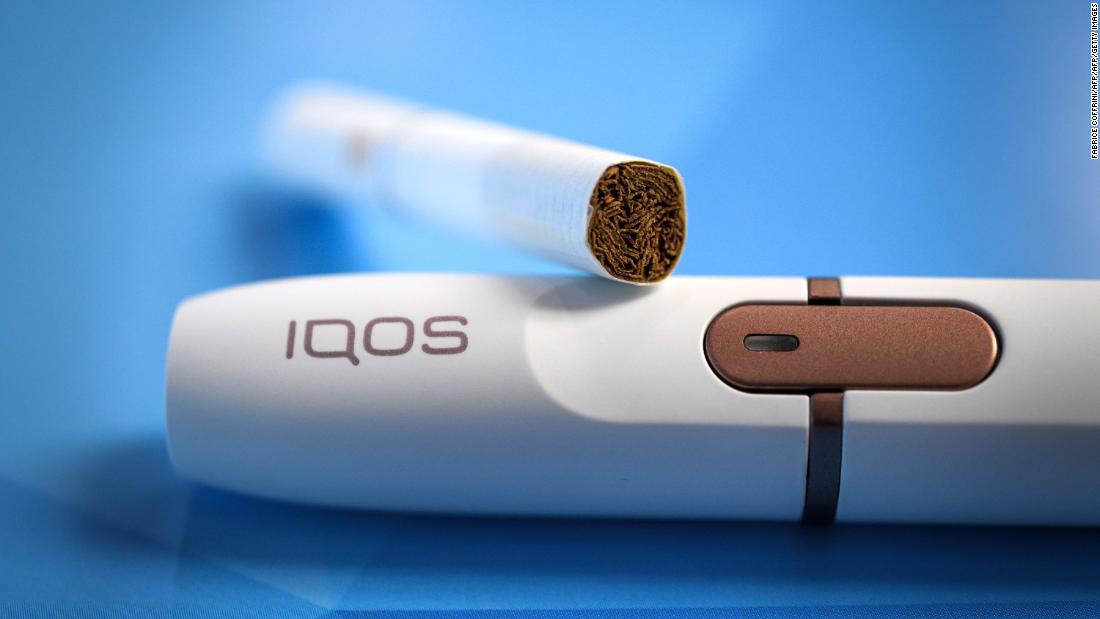
The agency said it did not consider the device – the Philip Morris International IQOS "tobacco heating system" – to be safe. And his decision to authorize sales does not amount to FDA approval.
The agency said the decision followed a "rigorous scientific review," after which it was decided that the product could have public health benefits, in part because the device contained "fewer chemicals." toxic than cigarette smoke, levels lower than in cigarette smoke. "
"Ensuring that new tobacco products are the subject of a strong pre-market assessment by the FDA is an essential part of our mission: to protect the public, especially young people, and to reduce the number of tobacco-related illnesses and deaths, "Mitch Zeller, director of the FDA's Center for Tobacco Products, said in a statement Tuesday.
Moira Gilchrist, vice president of strategic and scientific communications at Philip Morris International, said the FDA's decision was a "milestone" that would allow the company to market its product – sold in other countries – to American smokers.
The company hopes to distribute and market IQOS in approximately 90 to 120 days from Atlanta.
But the FDA's decision raised eyebrows among health advocates. Some fear that the product will spread among children, like vaping devices such as Juul.
He stated that his organization had repeatedly "expressed to the FDA its concerns that Philip Morris International had marketed IQOS in other countries in a way that would clearly appeal to children, especially via social media, sponsorship of events such as beach parties and fashion shows, as well as smooth shops and kiosks that look like, they sell technological gadgets, not tobacco products creating an addiction. "
Robin Koval, President and CEO of The Truth Initiative, said in an email that "the introduction of another nicotine delivery device to the US market while we are in the middle of a youth e-cigarette crisis is worrying ".
Harold Wimmer, president and CEO of the American Lung Association, said in a statement that he was "deeply concerned" about the potential effects of the product on health.
"The Lung Association urges the FDA to closely monitor how this product is actually used – and by whom," Wimmer said. "The FDA must take steps to prevent youth from becoming addicted to tobacco with this product."
The FDA said it would keep an eye on what will happen when the device comes on the market, including any adoption by young people. If this happens, the agency could reconsider its decision.
"We will be watching the market closely, including how the company will market these products," Zeller said. This involves respecting regulations to prevent children from being exposed to the product – on websites and social media, for example.
The agency will also require that the labels and ads on the packages warn of the dependence of the product. And the tobacco company can not market the devices "with claims of reduced exposure or reduced risk," the FDA said. To do this, the company has a separate application in the works.
The company's interest in smoke-free alternatives to cigarettes is part of a sector change. Last year, tobacco giant Altria took a 35% stake in Juul as part of a $ 12.8 billion deal.
Altria, who created Philip Morris International several years ago, will now distribute IQOS under an exclusive agreement with the company.
Danielle Wiener-Bronner from CNN contributed to this report.
[ad_2]
Source link
