[ad_1]
US regulators then took disciplinary action against vaping companies for inappropriately promoting their flavored nicotine formulas through so-called influencers on Facebook, Twitter and other sites. of social media.
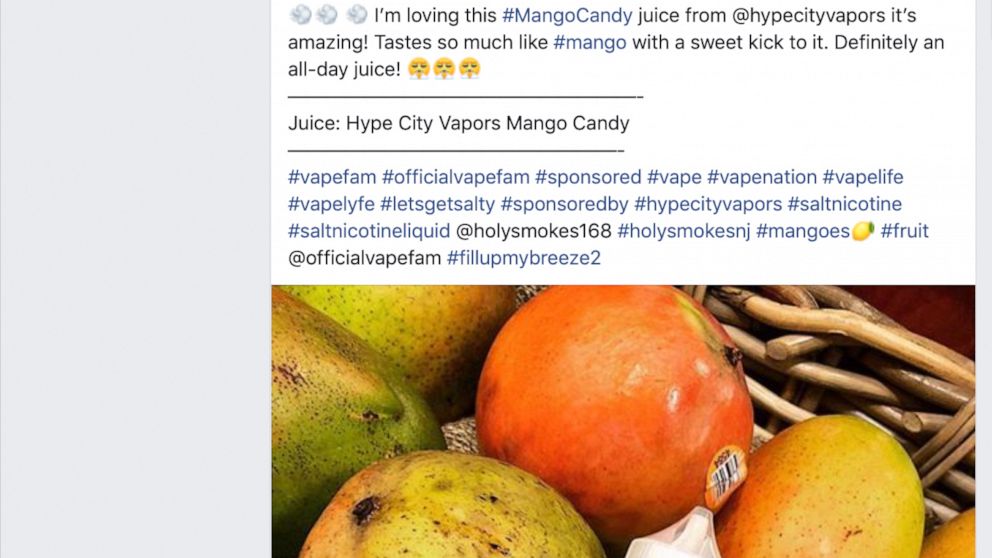
The Food and Drug Administration sent warning letters Friday to four companies that used influencers paid on social networks to present nicotine solutions to their online subscribers, including flavors like Watermelon Patch and Strawberry Kiwi.
The messages did not have a mandatory warning that vaping liquids contain nicotine, which is addictive. The FDA, along with the Federal Trade Commission, sent the letters to Solace Vapor, Hype City Vapors, Humble Juice Co., and Artist Liquid Labs. Businesses did not immediately respond to calls and emails requesting comments on Friday morning.
Facebook bans ads from e-cigarettes even with warnings and the FTC has been lobbying influencers – those who have many followers of social media and who promote products and services – to disclose when they are paid to endorse something .
This action comes as the FDA and other government agencies struggle to reverse what they call an epidemic of electronic cigarette consumption by minors. The researchers have associated the trend with an increase in the number of videos, photos and other online publications on vaping, generated for some by companies, advertising agencies and paid influencers.
Government figures showed an almost 80% increase in teenage vaping emissions last year, with one in five high school students reporting using the devices the previous month.
Electronic cigarettes generally heat a flavored nicotine solution in an inhalable aerosol. They are generally considered less harmful than traditional paper and tobacco cigarettes and some adult smokers use them as an alternative source of nicotine. But health experts warn that nicotine can hinder brain development and recent research shows that many teens are unaware that they consume the addictive chemical when they stuff themselves.
"It is essential that we ensure that manufacturers, retailers, and others include mandatory health warnings about nicotine addiction properties in packaging and advertising, especially on popular social media platforms. children, "said FDA Acting Commissioner Ned Sharpless.
The FDA's letters do not mention Juul, which dominates the US e-cigarette market. The Silicon Valley start-up is widely recognized for helping to trigger a vaping explosion with its early viral marketing, which included paid publications and recommendations from people influenced by social media.
After being scrutinized last year, Juul closed his Facebook and Instagram accounts in November. Since then, the company has redesigned its marketing to focus on older smokers who are interested in quitting.
In response to written questions from Senate legislators, Juul said in April that he currently does not use paid influencers on social media.
—
Follow Matthew Perrone on Twitter: @ AP – FDAwriter
—
The Health and Science Department of the Associated Press is receiving support from the Howard Hughes Medical Institute's Department of Science Education. The AP is solely responsible for all content.
[ad_2]
Source link