[ad_1]
New therapeutic option against eczema tested successfully
Atopic dermatitis is a chronic skin disease that has become more prevalent in recent decades. Although external treatments currently treat relatively benign forms of the disease, people with severe atopic dermatitis have little hope. This could soon change thanks to a new form of therapy.
"The disease affects about 11% of all pre-school girls and boys and 1% to 2% of adults in Germany.Many of the disease is chronic and difficult," reports Hannover Medical School (MHH) . Affected individuals suffer from dry, flaky and reddening skin that is itchy a lot and if the affected areas are clearly visible, a social stigma is added. Effective treatment options are therefore urgently needed – but they were not yet available for severe forms of the disease. However, researchers at the MHH and the University of Veterinary Medicine Hannover (TiHo) have successfully tested a new approach. Their findings were published in the Journal of Allergy and Clinical Immunology.
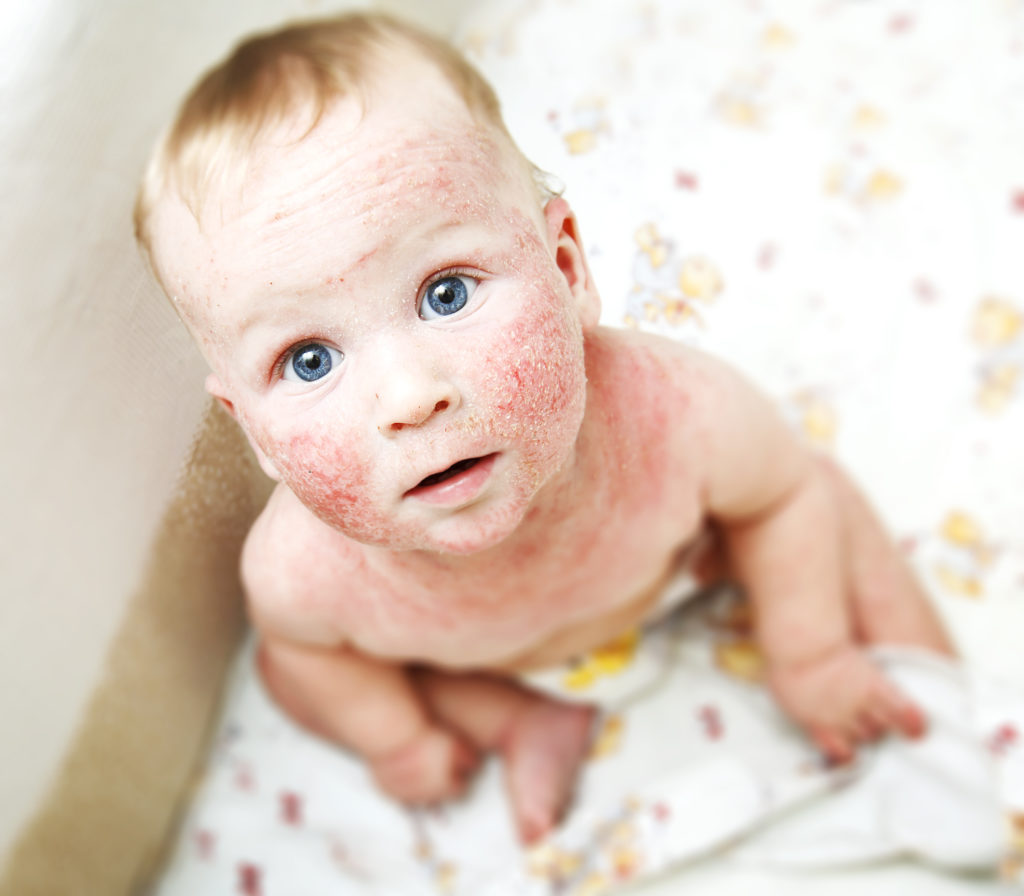
Severe eczema that is only partially treatable
"Atopic dermatitis has different causes, including irritants, allergens and microbial, hormonal and psychological influences," experts say. In the treatment of cortisone compounds and externally applied calcineurin inhibitors, they are of paramount importance. According to experts, only cyclosporine, an immunosuppressant with many side effects, and dupilumab antibody are available for the treatment of particularly severe forms.
Dupilumab slightly difficult to use
Dupilumab has been available for about a year for the targeted inhibition of allergic inflammation messengers and "represents a considerable advance in the treatment of critically ill patients," said Prof. Dr. med. Thomas Werfel of the MHH Clinic of Dermatology, Allergology and Venereology. However, it does not help all patients well enough. In addition, the drug must be injected, which is particularly difficult to tolerate for children who are particularly susceptible to eczema. The new drug, now tested, is however intended for oral use.
New active substance for oral use
The new active ingredient, which can be taken in tablet form, had significantly improved the appearance of the skin when tested on 98 patients. "Already after eight weeks, the proportion of diseased skin such as redness, blisters and scratches has been reduced by half," said the MHH. The drug is a "histamine-4 receptor blocker". This interrupts the process of inflammation and relieves the itching by preventing the messenger histamine from acting on the corresponding cells.
The histamine-4 receptor with a key role
"The laboratory and in vivo results in the murine model, which we have been releasing continuously since 2005, suggest that the histamine-4 receptor is an attractive target for the treatment of atopic dermatitis," says the professor. . Werfel. Since then, researchers have conducted extensive research on the use of inflammatory skin diseases. "We hypothesize that the histamine-4 receptor blocker acts independently of the cause of atopic dermatitis and are currently studying which patients might benefit the most from the new treatment," said Professor Werfel.
No side effects detected
According to the scientists, none of the side effects observed in this study are due to the administration of the drug. Now, with the participation of the Hanover team, a larger international study of approximately 400 patients will begin to look for the optimal dosage of this drug. "We have been working together on this topic for many years, and the project is a very good example of translational research, which is interdisciplinary medical research, with the goal of translating the results as quickly as possible into clinical applications," said Prof Dr. med. Manfred Kietzmann of the Institute of Pharmacology, Toxicology and Pharmacy of TiHo. (Fp)
Source link