[ad_1]

The World Health Organization has endorsed a new antiretroviral regimen. This follows preliminary results from studies including an ongoing trial in South Africa.
The ADVANCE study, conducted by a group of researchers from the University of the Witwatersrand based in Johannesburg, will be completed only next year. But the first results show that dolutegravir is an effective and well tolerated antiretroviral. ADVANCE, which will continue for 96 weeks, presented its results over a 48-week period at the International AIDS Conference in Mexico City.
The results are important because the Johannesburg trial includes a much more representative population of real-world populations treated for HIV in low- and middle-income countries. The study participants are blacks and nearly 60% of women of average age are 32 years old.
Previous studies on dolutegravir involved approximately 3,000 participants, most of whom were middle-aged white men from high-income countries in the United States and Europe. They clearly do not reflect the global and South African HIV epidemic, which is predominantly black (over 70%) and women (over 52%), many of whom are under 40 years of age.
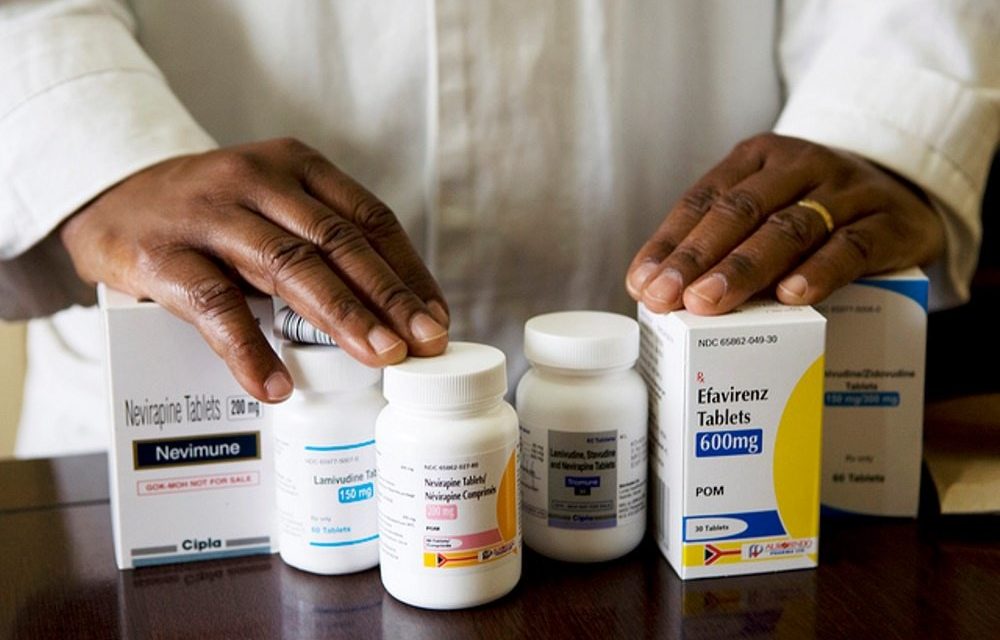
The drug efavirenz has been used for many years in the public sector antiretroviral treatment program in South Africa and has served the country well. But he has disadvantages. HIV becomes easily resistant; it is relatively expensive and it causes side effects in some people. This explains why dolutegravir is introduced into many HIV programs around the world, based on recent World Health Organization guidelines. Efavirenz will still be used.
There were also other gaps, including little or no data regarding the use of dolutegravir in people with advanced disease; pregnant or lactating women; people co-infected with HIV and TB; infants and children; and the elderly.
L & # 39; study
All participants (over 1000) of the ADVANCE study were recruited in downtown Johannesburg. Only 60% were South African, the remaining 40% from other parts of sub-Saharan Africa, mainly from Zimbabwe.
ADVANCE has been designed to be as inclusive as possible. It was intended to fill some of the gaps in the evidence left by the dolutegravir development program. These included:
do not restrict the number of CD4s;
allow participants who have developed tuberculosis or become pregnant during the course of the study to remain in the study;
recruitment from 12 years old; and
keep the exclusion criteria to enter the study to a minimum.
Another unique feature of ADVANCE is that it was designed by a consortium of leading international clinicians and researchers in the field of HIV. International organizations such as the World Health Organization, the Clinton Health Access Initiative, as well as advocates for treatment and activist groups have also contributed.
It was funded by USAID, Unitaid, the South African Medical Research Council. The drugs in the study were donated by Gilead Sciences and ViiV Healthcare.
What has been found so far
The ADVANCE study compares two antiretroviral drug regimens containing dolutegravir with a regimen of efavirenz in people starting antiretroviral therapy. He also compared the skeleton of ART containing tenofovir disoproxil fumarate (TDF) currently used with the new tenofovir alafenamide fumarate (TAF).
Early studies in Europe showed that dolutegravir did not have many side effects. Insomnia was the most marked side effects. In addition, dolutegravir and TAF are less expensive to manufacture on a large scale and can easily be converted into a single pill at a much lower dose than the fixed-dose combination currently used in the public sector.
The study showed that people who started ART with dolutegravir-based therapy had the same high rates of viral suppression as those starting with efavirenz. We also found very little treatment failure and, in most cases, even people with high viral load subsequently had an undetectable viral load after adherence counseling.
Very few people stopped the study because of side effects, although more people taking efavirenz experienced side effects.
Virtually none of the ADVANCE participants have developed tuberculosis – a common co-infection in people living with HIV – as almost all have received preventive treatment for TB.
But there are some problems. One of the findings was that we found weight gain in participants receiving dolutegravir treatment, which was worse for women and those who received both dolutegravir and TAF. Obesity carries many health risks in terms of the development of high blood pressure, risk of diabetes and other problems. For this reason, we monitor the levels of obesity among ADVANCE participants.
We do not know what the broader implications of this finding are. However, similar results regarding weight gain badociated with the use of integrase inhibitors, including dolutegravir, were presented at the March 2019 retrovirus and opportunistic infections conference. The findings emphasize that it is important to include screening for comorbidities and appropriate lifestyle interventions when treating affected individuals. HIV. Further research is needed to understand the cause and implications of observed weight gain with integrase inhibitors.
The number of pregnancies in ADVANCE is too low to allow meaningful conclusions to be drawn about the risk of neural tube defects. We have not seen any neural tube defects in the study.
Why is it important
ADVANCE is an important study. It confirms that dolutegravir is an effective ARV that is well tolerated by people starting antiretroviral therapy.
This is rebaduring as South Africa plans to deploy it as part of its antiretroviral program.
Because ADVANCE included a diverse African population and was much more representative of the demographics of the HIV epidemic, its results are important for both people living with HIV in South Africa and the world.
The findings were shared with the World Health Organization to inform discussions on guidelines and with regulators around the world.
Source link