[ad_1]
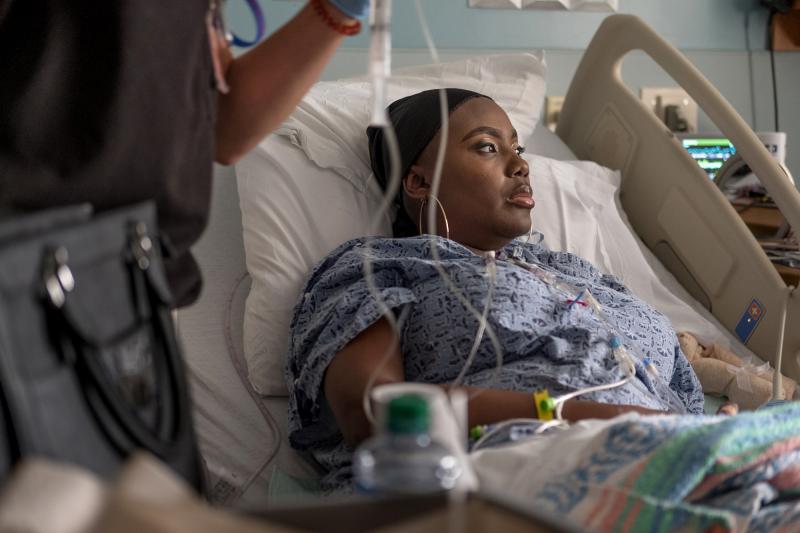
Victoria Gray patiently waits in a hospital room at the Sarah Cannon Research Institute in Nashville.
"It's a good time to heal," she says. The 34-year-old woman from Forest, Missouri, has struggled with sickle cell disease throughout her life.
Gray is in the hospital because she volunteered for one of the most awaited medical experiments in decades: the first attempt at using the modification technique CRISPR gene to treat a genetic disorder in the United States.
She is the first patient to have been publicly identified as involved in such a study.
"I had always hoped something would happen," Gray said in an exclusive interview with NPR. "It's amazing how much things have changed, I just want to help raise awareness of this disease and let others know that there is hope."
Sickle cell disease affects millions of people around the world. About 100,000 people are in the United States, and most of them, like Gray, are African-Americans. A genetic defect causes bone marrow production of a defective protein that forms sickle-shaped blood cells, hard and sticky. The deformed cells get stuck in the blood vessels and do not carry oxygen normally, resulting in a host of debilitating complications that often end up shortening life.
"It's horrible," Gray says. "When you can not walk or raise a spoon to feed yourself, it becomes very difficult."
For the study, doctors use cells taken from patients the bone marrow that will be genetically modified with CRISPR to enable them to produce a protein that is usually only produced by fetuses and babies for a short time after birth.
The hope is that this protein will compensate for the defective protein that causes sickle cell disease and allow patients to live normally for the rest of their lives.
"It's exciting to see that we could be on the cusp of developing an extremely effective treatment for sickle cell patients," said Dr. David Altshuler, Executive Vice President, Global Research and Scientific Director of Vertex Pharmaceuticals. Boston. Vertex co-sponsors the study with CRISPR Therapeutics of Cambridge, Mbad.
"People with sickle cell disease have long been waiting for treatments that simply allow them to live a normal life," said Altshuler.
"It's a very big deal," acknowledges Dr. Haydar Frangoul, medical director in pediatric hematology / oncology at the institute where Gray volunteered. "This could benefit many patients."
Frangoul's center, Sarah Cannon, is conducting the study at HCA Healthcare's TriStar Centennial Medical Center in Nashville, one of eight sites recruiting patients for research in the United States, Canada, and Europe. Up to 45 patients between the ages of 18 and 35 will eventually be included.
Other doctors, scientists and bioethicists are also encouraged.
"It's an exciting time in medicine, CRISPR promises to change the human genome and attack genetic diseases directly," says Laurie Zoloth, a bioethicist at the University of Chicago.
But Zoloth is also careful. It is concerned that this study and other studies on the use of CRISPR have not been thoroughly reviewed by a group of external experts convened by the National Institutes of Health.

"It's a brand new technology," says Zoloth. "It seems to work very well in animals and in culture dishes, it's totally unknown how it works in humans, so there are a lot of unknowns that could make you sicker."
Frangoul recognizes that experimental treatments always carry risks. But, he says, research will go very slowly and carefully with careful consideration from the Food and Drug Administration and other advisory groups.
"We are very cautious about how we conduct this test in a very systematic way to carefully monitor patients for any treatment-related complications," he said.
For her part, Gray says that she understands that there is risks. She also says that she knows that studying is a first step and that other patients might only see benefits in years.
"It gives me hope if it does not give me anything else," says Gray.
It will probably be several months before doctors detect the first signs of whether genetically modified cells produce useful levels of protein and even longer to know if cells improve the health of patients. And it will probably take several years to find out if the benefits will last a lifetime, as hoped.
Gray, married and a mother of four, was diagnosed with sickle cell disease when she was a child and started crying while bathing. A major symptom is excruciating and debilitating pain.
Like many sickle cell patients, her symptoms prevented her from living fully. She could not play like other children, was afraid to travel and had to give up her dream of becoming a doctor or nurse.

"Sometimes I get lightning in my chest and sharp pains everywhere," says Gray. "Sometimes I just curl up and cry, unable to do anything for myself."
Defective blood cells also increase the risk of infections and damage to vital organs such as the heart. They can also cause life-threatening strokes. Many people with sickle cell disease do not live after their forties. Gray's heart has already been damaged. And that takes an extra psychological toll.
"It's scary," she says, describing the effects of her illness on her eldest son, Jamarius, 12 years old.
"He is older, so he understands, so he started playing at school, and his teacher said:" I think Jamarius is acting because he really believes that you are going to die ", said Gray, stifling his tears.
Some patients may have bone marrow transplants, but these procedures are exhausting and can be dangerous if the immune system cells produced by the transplanted bone marrow attack their body. And most patients with sickle cell disease do not have or do not find a suitable donor.
"It's very difficult," says Gray. "It's just my religion that just kept me going."
When she was considering a bone marrow transplant, she heard about the CRISPR trial and jumped on the opportunity to volunteer.
"I always knew something had to happen and God was saving me something important," says Gray. "It was as if it was supposed to be – it was a little fate – it was an incredible feeling."
CRISPR enables scientists to make very specific changes to DNA, raising hopes for new methods of preventing and treating many diseases.
"CRISPR technology has many potential applications in the future, not just in blood diseases," says Frangoul.
Doctors have already started using it to try to treat cancer, mainly in China. In the United States, at least two patients have been treated for cancer, as part of a study conducted at the University of Pennsylvania in Philadelphia.
Later this year, Boston doctors plan to use CRISPR to edit cells in patients' retinas, hoping to restore sight in patients with inherited blindness.

Companies sponsoring the Sickle Cell Disease study announced earlier this year that they had used CRISPR to treat the first German patient with a similar blood disorder, beta-thalbademia.
Both beta-thalbademia and sickle cell disease are caused by genetic defects that cause problems with a protein called hemoglobin. Healthy red blood cells use hemoglobin to carry oxygen throughout the body.
The red blood cells of sickle cell patients carry defective hemoglobin that deforms them and does not carry enough oxygen. The hope is that another form of hemoglobin, called fetal hemoglobin, will offset the defective protein.
Fetal hemoglobin is produced by fetuses in the uterus to provide oxygen. In most people, the production of fetal hemoglobin stops shortly after birth.
"Once a baby is born, the switch will start," says Frangoul. "It's a gene that tells red blood cells – the bone marrow cells that produce red blood cells – to stop producing fetal hemoglobin."
This CRISPR treatment starts with doctors extracting bone marrow cells from patients' blood. The company's scientists then use CRISPR to modify a gene in the cells so that they produce fetal hemoglobin.
"This will help cells produce more fetal hemoglobin and make them happier and healthier," Frangoul said.

The patients then undergo the same type of grueling chemotherapy, administered as part of a standard bone marrow transplant. This eliminates existing cells carrying the genetic defect. But instead of receiving new cells from a donor, patients receive billions of their own cells that have been modified with CRISPR.
The hope is that he provide a treatment option for all patients, including those who can not find a compatible donor. I hope that the approach will be safer because the cells come from the patient's own bone marrow. So, they should not attack the patients' bodies, says Frangoul.
"This opens the door to many patients likely to be treated and modify their disease so that it is gentle" and to avoid "the horrible and horrible side effects of sickle cell disease," he says.
Gray hopes it works and describes how she imagines a life without sickle cell disease:
"Being able to wake up without hurting and just being tired because I've done something – and not just tired for no reason – and just to be out and jump on the trampoline with my kids.
"And go to graduation and weddings, and see them grow, that means the world to me."
9(MDA4MDYxNjY4MDEzMTQ2OTkxMzkyOWU2NQ004))
Source link