[ad_1]
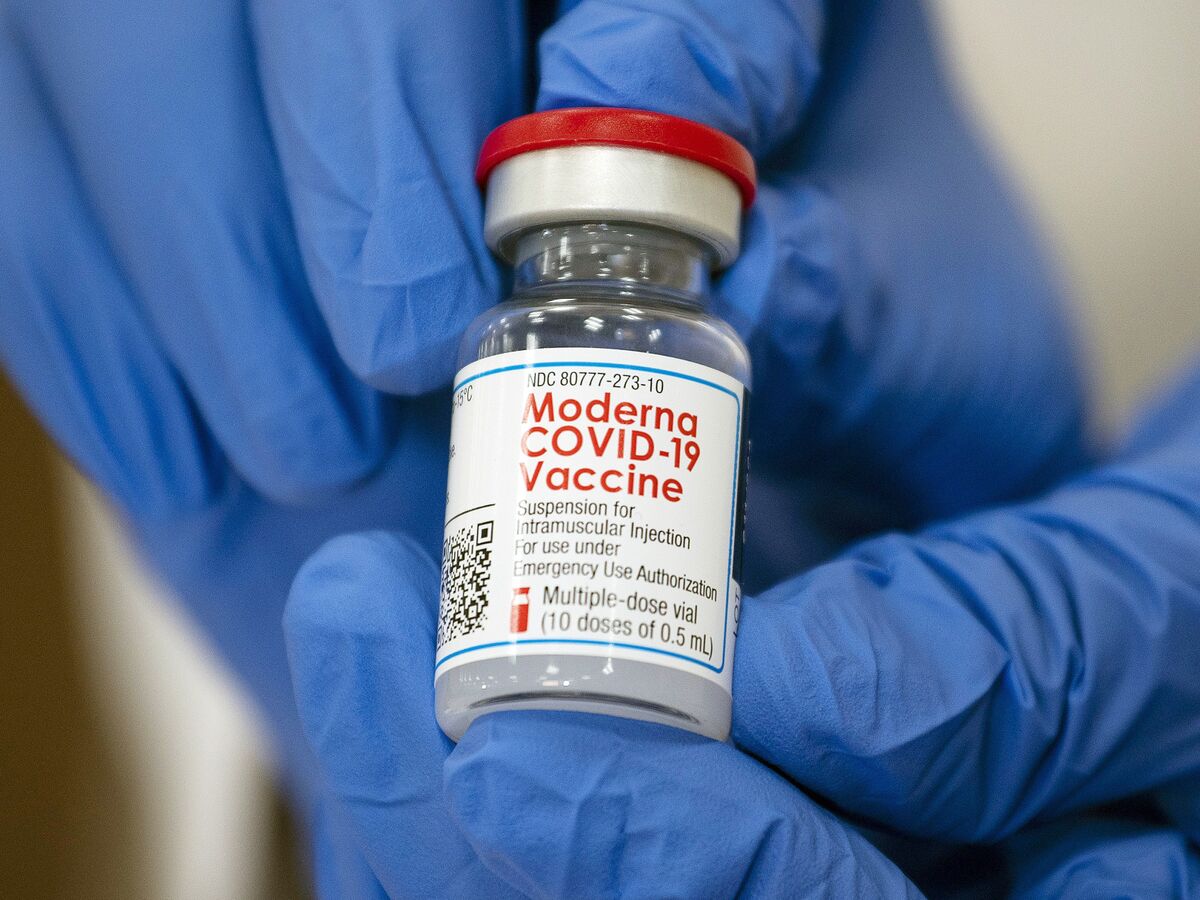
A vial of Moderna Inc. Covid-19 vaccine.
Photographer: Eduardo Munoz / Reuters / Bloomberg
Photographer: Eduardo Munoz / Reuters / Bloomberg
UK examines Moderna Inc.’s Covid-19 vaccine is on an accelerated schedule and is set to clear it for emergency use as early as Friday, according to people familiar with the situation.
The American company’s vaccine is said to be the third vaccine approved in the UK as the country ramps up its vaccinations against the rapidly spreading coronavirus and the infection rate rises. The death toll from Covid now stands at more than 78,000 across the country.
Over 17.5 million photos donated: Covid-19 Vaccine Tracker
Britain has ordered 7 million doses of the vaccine, but they are not expected to be delivered until later this year. AstraZeneca Plc and the University of Oxford’s Covid-19 shots were granted clearance from the UK late last month. This followed the authorization of another vaccine, Pfizer Inc. and BioNTech SE, early December. the The European Union and the United States have also authorized the Moderna product.
Moderna shares rose 1.6% in pre-market trading in the United States.
Prime Minister Boris Johnson anchored his hopes for a nationwide recovery on a plan to deliver 2 million coronavirus vaccines per week, after the UK returned to lockdown in an attempt to prevent hospitals are overwhelmed.
(updates with shares)
Source link