[ad_1]
The main goal of any vaccine is to stimulate immune function and provide long-lasting protection. An article recently uploaded to the pre-print server bioRxiv* by Tong et al. (17e February 2021) describes a new vaccine using PIKA adjuvant and advanced recombinant COVID-19 disease proteins that promises the generation of potent neutralizing antibodies, which potentially support immune responses for years.
What are Recombinant Vaccines?
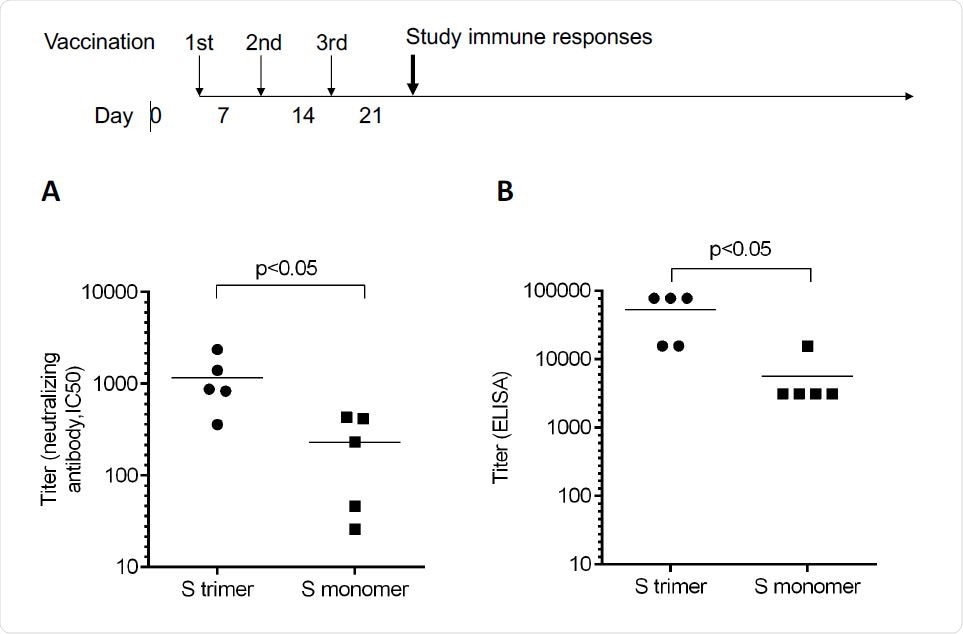
Recombinant proteins are created in genetically modified cells, modified to express the protein of interest. In this case, the group chose the full-length spike protein of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), as it contains many regions capable of inducing virus-specific T cells. The spike protein was modified by two mutations in proline to keep it in its pre-fusion form, a technique similar to that adopted by several other vaccine developing groups. PIKA was also incorporated into the final vaccine, and mice were administered weekly over a three week period with 5 μg of protein and 50 μg of PIKA. The group found that specific antibodies to the spike protein were detected at a titre of over 50,000 at the day 21 peak, with an average of 1,000, supposedly higher than that achieved by the three approved vaccines (Moderna, Oxford- AstraZeneca and Pfizer -BioNTech).
Polyinosinic: Polycytidylic acid (poly I: C) is a synthetically produced unpaired dsRNA strand that interacts with toll-like receptors on the surface of immune cells to act as an antiviral agent. It is generally referred to as PIKA when used as a vaccine adjuvant in a stabilized form and has been shown to provide quantitative and qualitative improvements in immune responses when administered concomitantly with an unadjuvanted vaccine.

What are the advantages of recombinant vaccines?
A protein / peptide array was made to test the binding method and showed that the antibodies produced that bind to the modified spike protein do so in the same way as the natural spike protein, mainly around the binding domain. to the receiver. This demonstrates that the recombinant protein is able to stimulate the immune system similar to the real virus, conferring effective and lasting immunity.
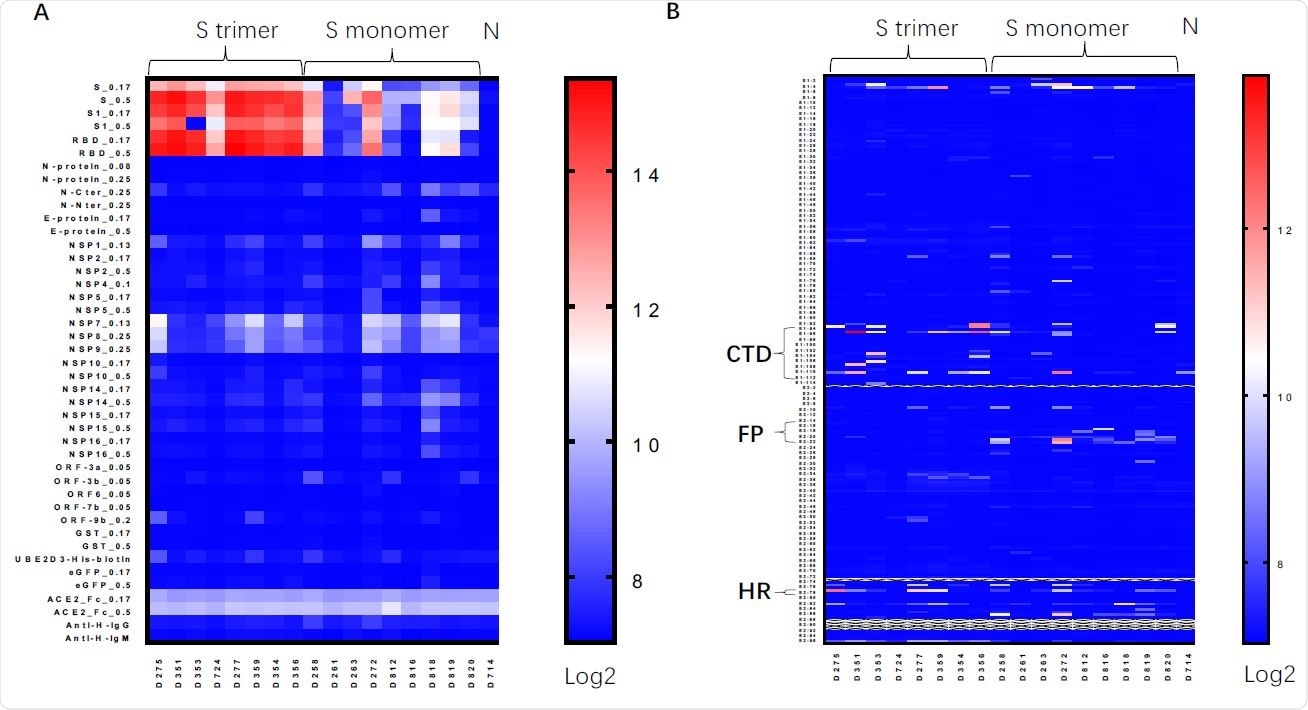
Table of serum antibody proteins / peptides induced by Spike protein vaccines. A. Protein matrix assay for sera from mice immunized with Spike trimer, Spike monomer, unimmunized mice, using Spike (S_0.17 and S_0.5 means proteins were imprinted at 0.17 or 0.5 mg / ml); S1 subunit of Spike, RBD and other viral proteins. B. Linear peptide array using linear peptides of Spike proteins. CTD, C-terminal domain just after RBD (Peptides S1-93-S1-113); FP, fusion peptide (Peptides S2-14-S2-23); HR, heptad regions (Peptides S2-78).
The group used Chinese hamster ovary (CHO) cells to express the recombinant protein. CHO cells are commonly used in the production of recombinant proteins because they are easy to cultivate on a large scale and produce prodigious amounts of the desired protein, up to 10 grams per liter of culture. Many decades of work with these cells have enabled detailed genetic engineering that produces human biocompatible molecules.
Recombinant protein and mRNA vaccines require the use of adjuvants, such as PIKA, in this case, to stimulate a robust immune response and confer long lasting immunity. However, recombinant protein vaccines do not need to be stored at -80 ° C, as is the case with the two most popular COVID-19 mRNA vaccines from Moderna and Pfizer-BioNTech. Other recombinant protein vaccines have been successful with longer lasting immunity than that conferred by mRNA vaccines, including hepatitis B and HPV vaccines. Comparatively, manufacturing is continuous, more easily scalable, and less expensive.
*Important Notice
bioRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behaviors, or treated as established information.
Source link