
[ad_1]
The European Union aims to vaccinate 70% of its adult population by September. But concern over a possible link between the Oxford-AstraZeneca vaccine and reported cases of blood clots may put that target at risk.
The deployment of vaccines in Europe has already been slower than expected. Meanwhile, the continent is facing a third wave of the pandemic, fueled by variants of the virus.
European heavyweights Germany, France and Italy – all of which have seen a recent surge in coronavirus cases – were among more than a dozen countries to suspend their deployment of the OxfordAstraZeneca fire while the European medicines regulator, the European Medical Authority (EMA), was investigating the concerns.
The EMA “came to a clear scientific conclusion. It is a safe and effective vaccine, ”Executive Director Emer Cooke said Thursday.
She said the group had not discovered that the vaccine caused clotting, although it could not definitively rule out a link with a rare blood clotting disorder.
Cooke added that the benefits of the vaccine outweighed the risks, a message already highlighted this week by the EMA and the World Health Organization (WHO).
Italy, France, Germany, Spain, Cyprus and the Netherlands have all announced plans to resume AstraZeneca vaccinations, more countries are expected to follow.
Milan’s largest vaccination center told CNN it will overbook appointments to try to make up for the shortages in recent days.
People line up to receive a stroke from AstraZeneca at a Rome convention center, temporarily turned into a Covid-19 vaccination center, on Friday, March 19.
But it’s unclear whether the EMA’s findings will do much to allay public concerns after a scorching week in the EU’s vaccination campaign.
Norway, Denmark and Sweden have all said they will keep AstraZeneca vaccinations on hold for now. And reluctance to vaccinate is already high in some countries, especially in France, where Covid cases are on the increase.
An online poll conducted March 15-16 by Elabe for CNN subsidiary BFM TV suggests that only 20% of French people surveyed trust the AstraZeneca vaccine.
While the French health authority gave the green light for the resumption of deployment of the AstraZeneca vaccine on Friday, it recommended its use only for people aged 55 and over, a document sent to CNN said, based on the fact that “Almost all” reports of blood clots leading to the suspension were in people under the age of 55.
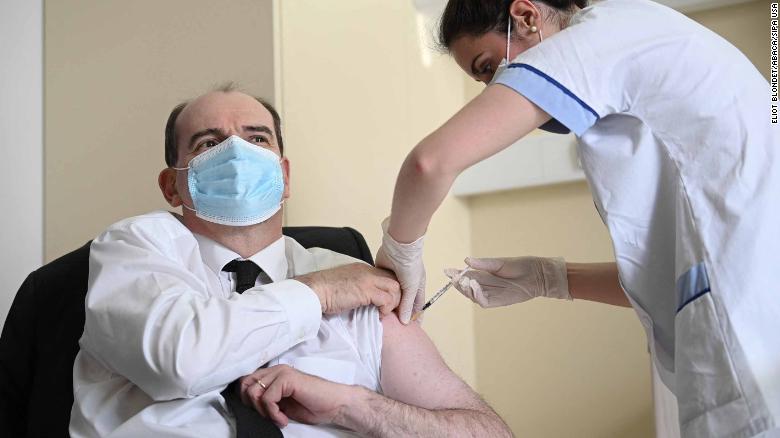
French Prime Minister Jean Castex, 55, rolled up his sleeve for an AstraZeneca shot on Friday in an attempt to reassure his compatriots about safety, as did British Prime Minister Boris Johnson.
But a senior Parisian hospital official, Remi Salomon, said this week he feared the impact of AstraZeneca’s suspension on vaccine confidence in France.
“Maybe people are being too careful,” Salomon told BFM TV. “My fear is that we are in France where a lot of people are reluctant to get vaccinated, I would say almost provocative, I’m afraid that people will not interpret this in the right way.
Michael Head, a senior researcher in global health at the University of Southampton in the UK, said the decision to take a break from AstraZeneca could “have a serious ripple effect, in terms of confidence and reluctance to lure. ‘regard to vaccines and adoption beyond that. “
“It takes a while to build confidence in a vaccine that we [the global health community] done with rigorous testing, with very good safety data, by being open and transparent about what we did and didn’t find, ”he told CNN, in an interview conducted before the release of the results of the EMA.
“When we have a widespread withdrawal of the vaccine in several countries, in some countries that are quite reluctant to vaccinate anyway, it can take a long time to restore that confidence.”
There is then a risk that people will start to favor one vaccine over another and thus delay inoculation to wait for the one they prefer, Head added, although the data on safety and effectiveness are fairly similar for all approved vaccines.
“We don’t want that to happen. I’m afraid that will happen. And if people are waiting for another vaccine or choose not to be vaccinated at all, then the pandemic lasts longer than necessary, ”he said. “Obviously, you will see more cases of Covid-19 and hospitalizations and deaths as a result.”
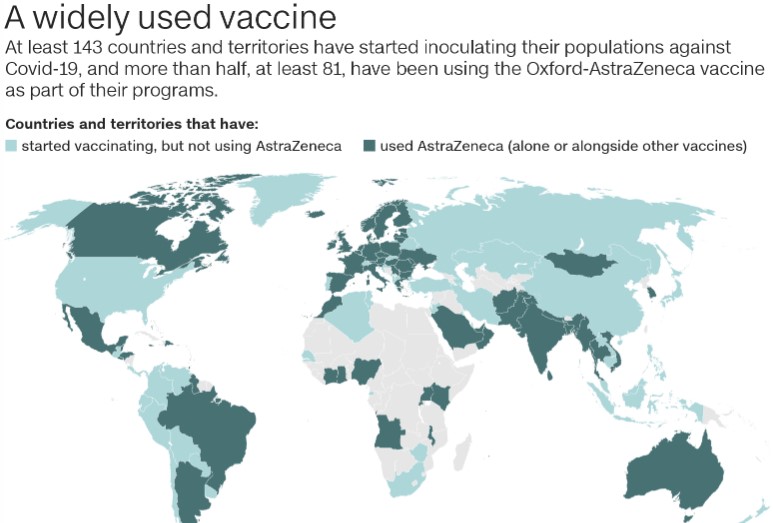
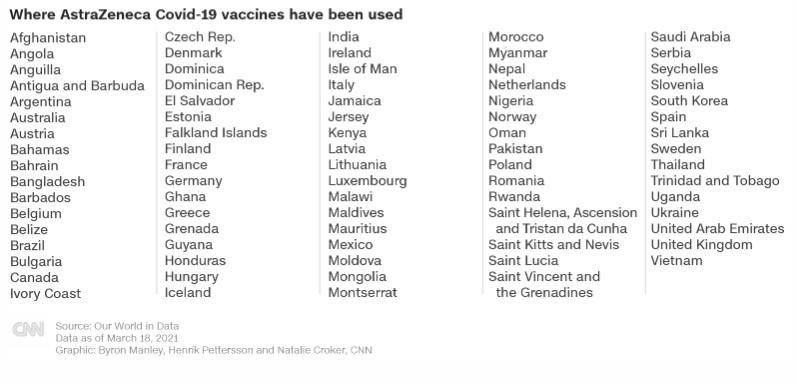
Source link