.jpg)
[ad_1]
New preclinical research led by Wensheng Wei of Peking University in China has proposed the development of a circular RNA vaccine. Their findings showed that the circular RNA vaccine creates neutralizing antibodies and strong T cell responses against the spike protein receptor binding domain of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
The circular RNA vaccine also effectively neutralized the mutated receptor binding domain found in the B.1.351 variant. The B.1.351 variant was originally discovered in South Africa and may escape the immune system.
Currently approved messenger RNA (mRNA) vaccines from Moderna and Pfizer-BioNTech were developed before the emergence of variants, so information is limited. Johnson & Johnson’s single-injection vaccine was effective against the original strain in Wuhan, China, and the B.1.1.7 variant was first reported in south London last fall.
The results could help treat variant B.1351 and other variants of concern.
The authors write:
“Since the SARS-CoV-2 variants encoding E484K or N501Y or K417N-E484K-N501Y escape certain neutralizing antibodies induced by mRNA vaccines, we anticipated that the effect of the hACE2 decoy encoded by the Circular mRNA might not be affected by viral mutations.
The study “Circular RNA Vaccines against SARS-CoV-2 and Emerging Variants” is available as a pre-print on the bioRxiv* server, while the article is subject to peer review.
.jpg)
Development of the circular RNA vaccine
The team used a group I ribozyme autocatalysis strategy to produce circular RNA-encoding antigens specific for the SARS-CoV-2 receptor binding domain (circRNARBD). A signal peptide sequence was added to the N-terminus of the receptor binding domain due to its secretory expression of antigens.
The IRES element was positioned before the sequence encoding the receptor binding domain to begin translation. An IRES-SP-RBD-T4 sequence was added to the cyclic vector to produce circRNARBD.
Circular RNA model shows high protein expression and thermal stability
Their circular RNA model was found to be more resistant to RNase R than to linear RNA. When the purified circRNARBD was placed in HEK293T cells, they found many antigens – 50 times more than linear RNARBD groups specific to the SARS-CoV-2 receptor binding domain. These antigens prevented infection with the SARS-CoV-2 pseudovirus.
They also showed high thermal stability. When stored at room temperature prior to transfection into HEK293T cells, circRNARBD continued to show expression two weeks after storage.
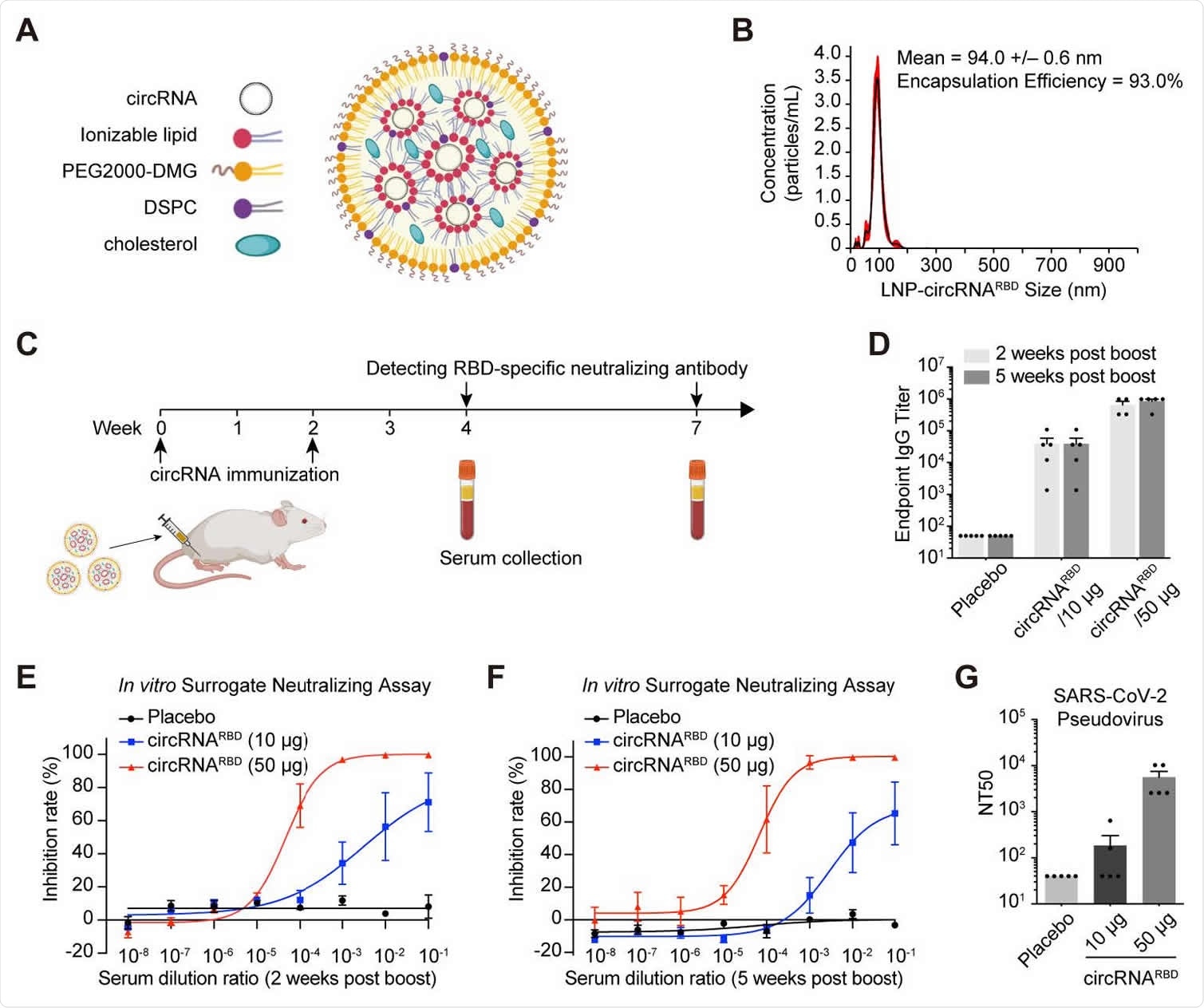
Robust immune response in mice
Mice were injected intramuscularly with 10 μg or 50 μg of experimental circRNARBD vaccine at two week intervals. Immunity was measured two to five weeks after their booster injection.
The titers of immunoglobulin G (IgG) and antibodies were expressed in a dose-dependent manner and persisted for two and five weeks after their booster injection. It also effectively neutralized a SARS-CoV-2 pseudovirus. Researchers suggest circRNARBD the vaccine creates a lasting immune response against SARS-CoV-2.
When assessing CD4+ and CD8+ T cell immune responses after vaccination, researchers found biased Th1 responses that produced interferon-γ (IFN-γ), tumor necrosis factor (TNF-α), and interleukin-2 ( IL-2). However, no modification of interleukin-4 (IL-4) was observed. The results of the circular RNA vaccines stimulated a Th1 immune response but not a Th2.
There were also several cytokine-producing CD8s+ detected in circRNARBD vaccinated mice. Interestingly, the team also found more robust immune responses in CD4 + and CD8 + effector memory T cells at 10 mcg than 50 mcg. However, 50 μg induced higher potency of neutralizing antibodies in B-cell responses.
The circular RNA vaccine is effective in neutralizing the B.1.351 variant
The team collected serum from immunized mice 1 and 2 weeks after their booster injection. They found IgG titers specific to the spike protein receptor binding domain with the 501.YV2 mutation.
The researchers then evaluated the neutralizing activity of the mice with the circRNARBD or circRNARBD-501Y.V2 vaccines against the D614G, B.1.1.7 / 501Y.V1 or B.1.351 / 501Y.V2 variants. The circRNARBD the vaccine antibodies effectively neutralized the three viral strains with the highest activity against the D614G strain.
In contrast, the circRNARBD-501Y.V2 also neutralized all strains, with the highest neutralization activity against the corresponding variant, 501Y.V2.
“It should be noted that the two vaccines could neutralize all three strains, although with varying efficacy. Nonetheless, multivalent vaccines should have offered better protection to both the native strain of SARS-CoV-2 and its outstanding variants, ”the research team wrote.
*Important Notice
bioRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behaviors, or treated as established information.
Source link