.jpg)
[ad_1]
Researchers in the United States have demonstrated the effectiveness of three antibody agents in neutralizing recently emerged variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the causative agent of the 2019 coronavirus disease pandemic (COVID-19).
The team showed that one full-length antibody and two antibody domains each effectively neutralized the pseudotyped virus containing key mutations found in the B.1.1.7 variant that emerged in the UK.
However, only one of the antibody domains was effective in neutralizing pseudotyped virus containing key mutations found in the South African B.1.351 lineage.
“The results inform the further clinical development of these antibodies against emerging variants of SARS-CoV-2, either by making antibody cocktails or by building bispecific antibodies,” writes Zehua Sun and colleagues at the medical school. from the University of Pittsburgh in Pennsylvania.
A pre-printed version of the research paper is available on the bioRxiv* server, while the article is subject to peer review.
.jpg)
Study: Neutralization of European, South African and American SARS-CoV-2 mutants by human antibody and antibody domains
Emerging variants threaten current therapeutic approaches
Transmission of the recently emerged variants of SARS-CoV-2 remains unchecked in many countries. Studies have shown that monoclonal antibodies (mAbs) and vaccine-induced antibodies are less effective in neutralizing these variants, including the British line B.1.1.7, the South African line B.1.351 and the Brazilian line P .1.
The initial phase of the SARS-CoV-2 infection process is mediated by the viral spike protein. The receptor binding domain (RBD) of the tip binds to the angiotensin converting enzyme 2 (ACE2) of the human host cell receptor to allow viral fusion and entry into the cell.
Researchers have developed mAbs and vaccines that cause antibodies that neutralize infection by binding antibodies to the RBD peak to prevent its interaction with ACE2.
However, a number of mutations have been characterized in the new variants which prevent these neutralizing antibodies from binding to the RBD peak.
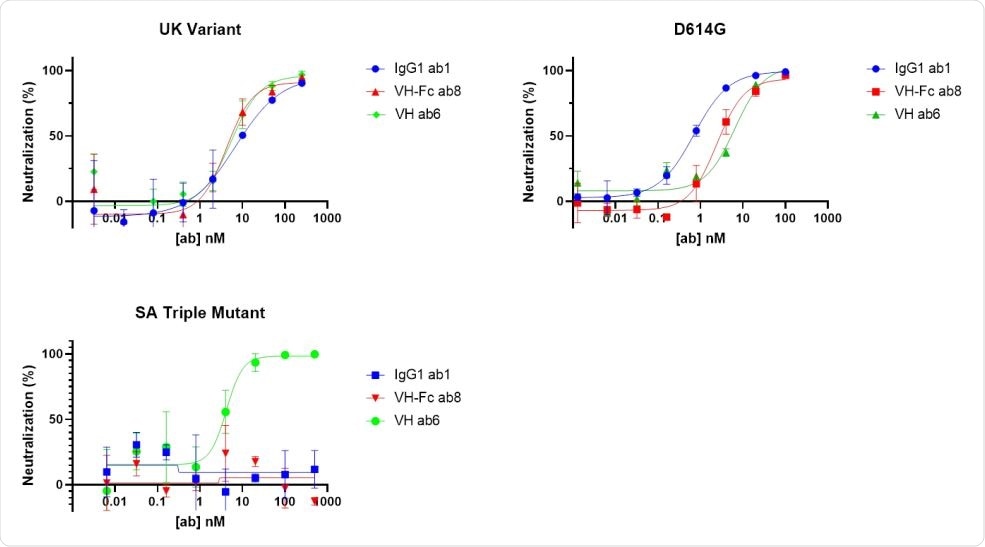
Neutralization test of our antibodies against pseudo-typed viruses carrying S proteins carrying different mutations.
How many mutations in the spike and RBD have the different variants evolved?
The British variant contains eight spike mutations, one of which (N501Y) is located in RBD, while the South African variant contains nine spike mutations, with three (K417N, E484K and N501Y) located in RBD. The Brazilian variant also contains this triple mutation (K417T, E484K and N501Y) in RBD.
“These mutations contribute to an increased affinity of RBD for human ACE2, thereby improving viral entry into cells,” explains Sun and colleagues. “The rapid emergence of new variants is of great concern due to their increased transmissibility and evasion of neutralizing antibody treatments and the humoral response generated by currently approved vaccines.
Additionally, although some of the mutations in these variants only partially reduce neutralization by vaccinated sera or currently approved mAbs, the ever-evolving variants could become dominant and more effective at evading therapies and vaccines, the team adds. .
Researchers previously identified a full-length immunoglobulin 1 antibody (ab1) and two antibody domains (ab6 and ab8) that exhibited potent neutralizing activity against the RBD of the wild-type SARS-CoV-2 spike protein. .
“Ab1, ab6 and ab8 are all antibody or antibody domains that can compete with human ACE2 to bind to wild-type SARS-CoV-2 RBD,” they write.
What was the current study?
Researchers evaluated the effectiveness of these previously identified antibody agents to bind and neutralize variants of SARS-CoV-2. They performed an ELISA against a panel of RBDs containing key mutations and performed pseudotyped viral neutralization assays.
The team found that the ab6 antibody domain bound to 32 of the 35 RBD mutants tested in the ELISA, including the triple mutation (K417N, E484K and N501Y) found in the South African and Brazilian variants.
However, this triple mutation completely blocked binding by the full-length ab1 antibody and the domain of the ab8 antibody.
“These observations indicate that ab1 and ab8 could completely lose the neutralization of the B.1.351 variant,” the team explains.
Ab6 also neutralized pseudotyped viruses with spike proteins containing B.1.1.7 mutations and spikes containing B.1.351 mutations.
In contrast, ab1 and ab8 only neutralized the B.1.1.7 variant and not the triple mutant variant.
What do the authors conclude?
“The domain of the ab6 antibody has shown potency in neutralizing all pseudotyped variants carrying these mutations,” the team explains.
Researchers say vaccination is still an effective approach for long-term control of transmission of the SARS-CoV-2 variant.
However, “our study provides very potent candidate antibodies that neutralize emerging mutant variants that can be used as emergency therapeutics where current vaccines are not present or less effective in prevention,” Sun and colleagues conclude.
*Important Notice
bioRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behaviors, or treated as established information.
Source link