[ad_1]
On March 13, 2020, the World Health Organization (WHO) officially declared the outbreak of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), which is the virus responsible for 2019 coronavirus disease ( COVID-19), be a global pandemic.
SARS-CoV-2 is a single-stranded ribonucleic acid (RNA) beta-coronavirus that shares 95% genomic sequence similarity with SARS-CoV-1, which was a coronavirus that spread to many countries in 2002 Scientists around the world are working tirelessly to develop effective vaccines to contain the COVID-19 pandemic.
Previous research has indicated that the spike (S) protein is an important target for emerging vaccines, as well as for therapies. To date, most COVID-19 vaccines have been designed based on the entire S antigen or a truncated form that can induce host immune responses and protect the body against viral infection.
Several vaccines, based on nucleic acid or adenovirus technologies, have received emergency use authorization in many countries around the world. Vaccinating the world’s population will require billions of doses.
 Study: The one- or two-dose regimen of the SARS-CoV-2 INO-4800 synthetic DNA vaccine protects against the respiratory disease burden in the non-human primate challenge model. Image Credit: Billion Photos / Shutterstock.com
Study: The one- or two-dose regimen of the SARS-CoV-2 INO-4800 synthetic DNA vaccine protects against the respiratory disease burden in the non-human primate challenge model. Image Credit: Billion Photos / Shutterstock.com
INO-4800: a synthetic DNA vaccine
INO-4800 is a codon optimized plasmid DNA vaccine that encodes the wild type SARS-CoV-2 S protein. Earlier in vivo studies have shown that INO-4800 can elicit T and B cell responses against SARS-CoV-2. Scientists have found that this vaccine can also reduce viral replication when challenged by mice transduced with human angiotensin converting enzyme 2 (ACE2).
Likewise, inoculation of INO-4800 into the rhesus macaque SARS-CoV-2 challenge model showed the induction of immunological memory cells that could reduce viral replication. For the development of vaccines and therapeutics, the model of non-human primates (PNH) is extremely valuable, as these animals can be infected with SARS-CoV-2 and develop symptoms similar to those of humans with COVID- 19 light.
How effective is INO-4800?
The Phase I clinical trial of INO-4800 vaccine demonstrated its safety and tolerability profile. In fact, this vaccine elicited immune responses in 100% of recipients and was found to protect these individuals by inducing both SARS-CoV-2 neutralizing antibodies as well as cellular immune responses.
Currently, the INO-4800 vaccine is undergoing further evaluation as part of a Phase 2 segment of a Phase 2/3 study. The main objective of this clinical trial is to further assess the safety, immunogenicity and efficacy of the vaccine.
A new study published in Vaccine is evaluating the safety and efficacy of the synthetic DNA vaccine candidate INO-4800, administered as a single or double dose regimen. This vaccine was administered into the skin of the rhesus macaque using CELLECTRA-ID electroporation technology.
In this study, 12 rhesus macaques, including 6 males and 6 females, were vaccinated with the INO-4800 vaccine. One group received a dose of the injected vaccine on day 28, while the second group received a dose of the vaccine on day 0 and a second dose on day 28. Serum titers of SARS antigen-reactive immunoglobulin G -CoV-2 S (IgG) antibodies were measured in all animals every two weeks between days 0 and 56. Scientists reported that this vaccine elicited a functional T cell response because antibodies neutralizing the SARS protein -CoV-2S were enhanced after the second dose.
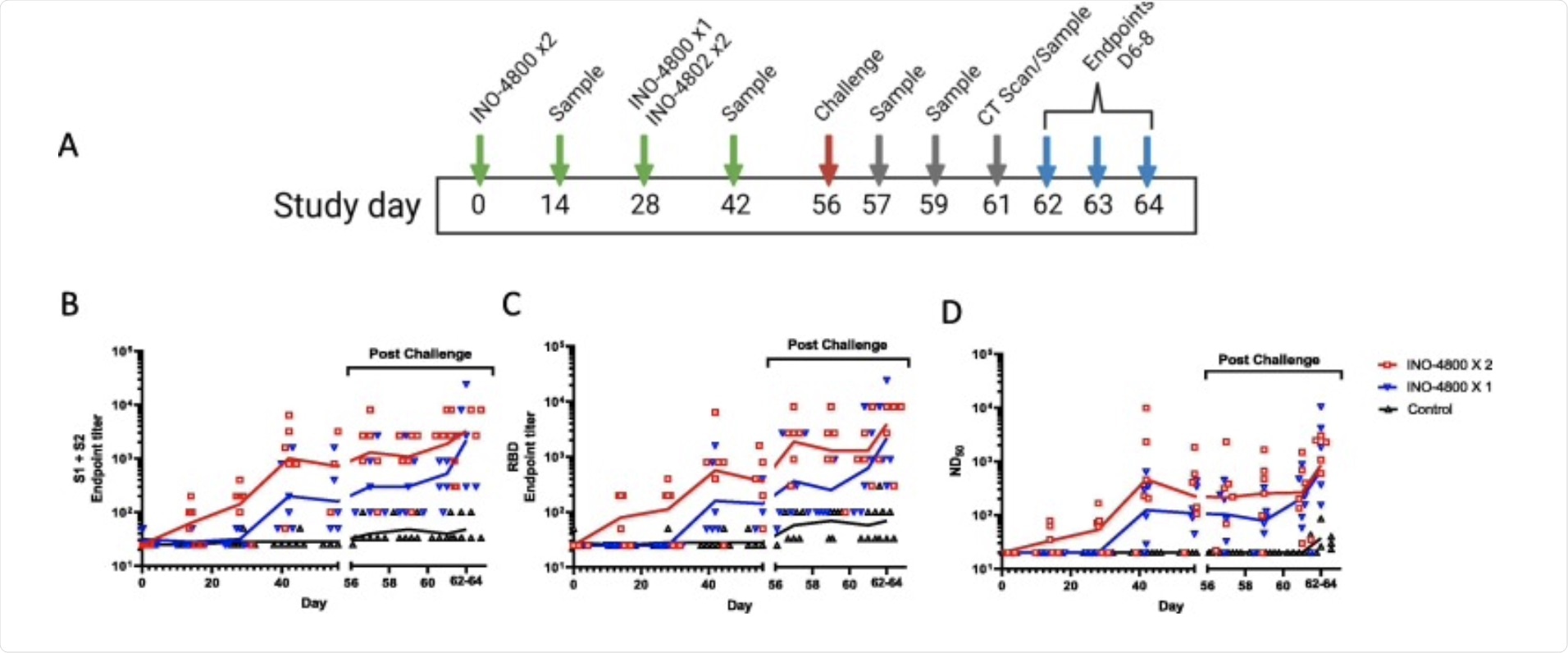 Humorous responses in rhesus macaques vaccinated with INO-4800. Study plan (A). SARS-CoV-2 Spike-specific IgG (B), RBD-specific IgG (C), and live virus neutralizing antibodies (D) were measured before and after challenge in serum from rhesus macaques given 1 or 2 doses of INO-4800 or were not vaccinated (Control). The lines represent the geometric means.
Humorous responses in rhesus macaques vaccinated with INO-4800. Study plan (A). SARS-CoV-2 Spike-specific IgG (B), RBD-specific IgG (C), and live virus neutralizing antibodies (D) were measured before and after challenge in serum from rhesus macaques given 1 or 2 doses of INO-4800 or were not vaccinated (Control). The lines represent the geometric means.
The effectiveness of INO-4800 after the SARS-CoV-2 challenge
The vaccinated animals were then subjected to a high dose of SARS-CoV-2 strain Victoria01 (5X106 pfu) on the 56th day of their vaccination to assess the efficacy of the vaccine.
No significant clinical symptoms were observed throughout the study. Nose and throat samples were taken to measure viral RNA and subgenomic RNA (sgmRNA) content via quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) assay. This analysis revealed a significant negative correlation between nasal viral loads and neutralizing and anti-receptor (RBD) IgG titers on day 3, but not on day 1.
At the time of the autopsy, the researchers collected bronchoalveolar lavage fluid (BAL) from each animal. Analysis of these samples showed an overall reduction in SARS-CoV-2 viral RNA and sgmRNA levels in the vaccinated group. However, their levels varied depending on the day of the autopsy.
RT-qPCR was also performed on tissue collected at autopsy. This analysis revealed that in most tissues except the lungs, SARS-CoV-2 RNA levels were below the limit of quantification. The estimation of the viral load in the lung tissues showed a reduced viral load in the vaccinated animals.
Taken together, the RT-qPCR viral load data revealed a positive effect on reducing viral loads in rhesus macaques vaccinated INO-4800, even after being subjected to a high dose of SARS-CoV-2. In addition, histopathologic examination of the lung tissue did not provide any indication of vaccine-intensified disease in the vaccinated animals which were subjected to a high viral load.
Conclusion
The authors of this study conducted preclinical animal model SARS-CoV-2 studies to evaluate the INO-4800 vaccine. Their evaluation revealed that a single dose of the vaccine had a positive impact on lowering the viral load in the lungs and that no disease improved by the vaccine was found.
As synthetic DNA vaccines can be developed at a faster rate than conventional vaccines, they could play an important role in bringing the ongoing COVID-19 pandemic under control.
Source link