[ad_1]
Your saliva may be able to tell you if you’ve developed antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a newer suggests. medRxiv* pre-printing study. In immunocompromised groups, levels of salivary immunoglobulin G (IgG) antibodies were associated with IgG titers in blood serum 3-4 weeks after mRNA vaccination.

Immunocompromised patients develop weaker antibody responses than healthy individuals. As a group at high risk of developing severe COVID-19 infection, they require regular monitoring.
“The magnitude of anti-spike IgG responses in the saliva of vaccinated individuals, which exceeded those seen in mildly convalescent individuals, is encouraging as it indicates that the vaccination may confer a sterilizing immune response in the oral cavity and thereby reduce the transmission of the virus, “the researchers conclude.
Based on the results of the study, collecting saliva samples may provide a safe and convenient way to assess the immunity of this vulnerable population.
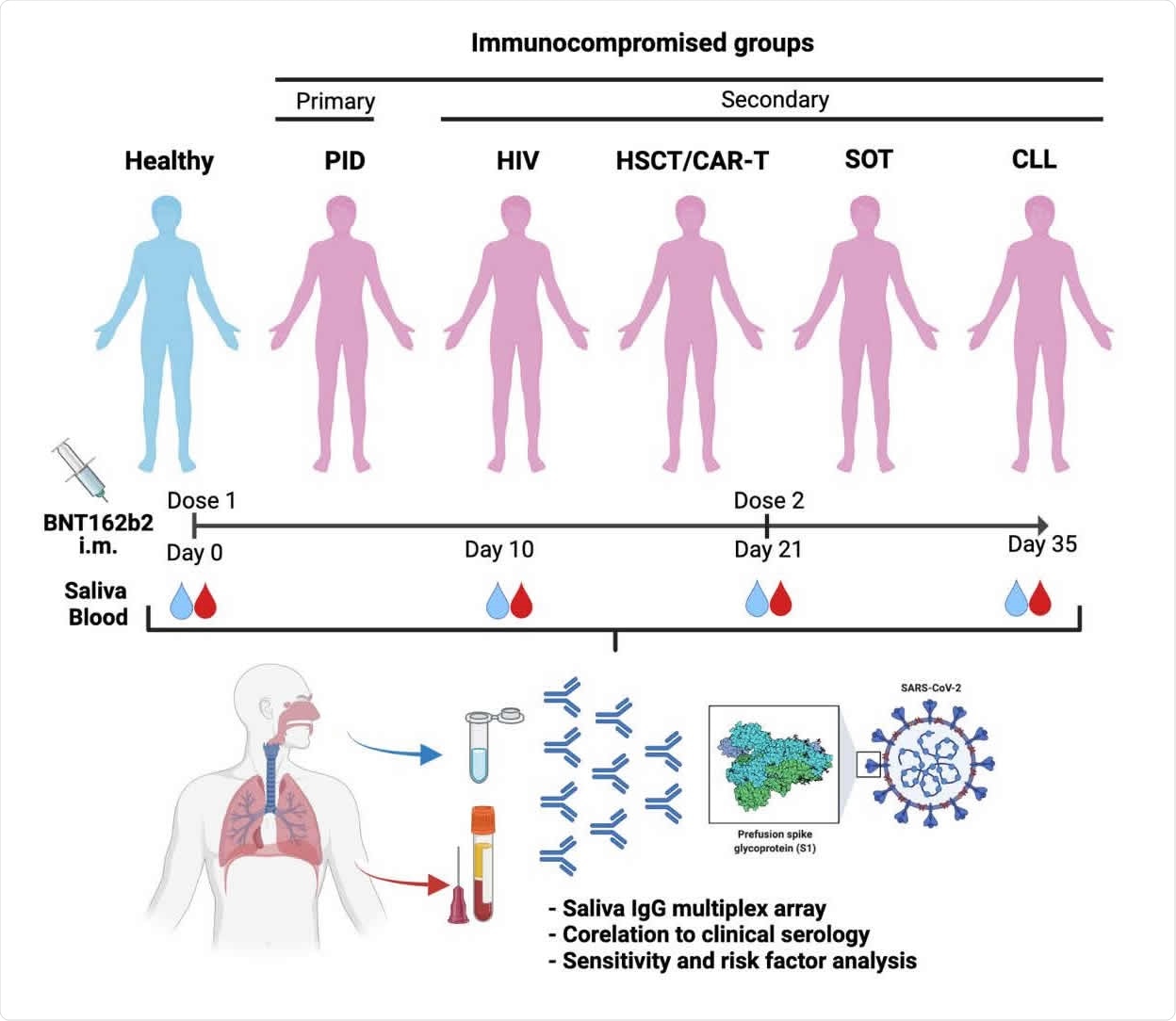
How they did
About 404 immunocompromised patients and 82 healthy participants participated in the study. At the time of the research study, it was confirmed that not all participants had antibodies to SARS-CoV-2 in their blood and did not test positive for SARS-CoV infection. -2.
All patients received two doses of Pfizer-BioNTech vaccine. Saliva and serum samples were taken before vaccination, 10 days after, 21 days after and 35 days after the first dose. The second dose of vaccine was given 3 weeks after the first dose.
IgG levels were measured because previous research suggested that the Pfizer-BioNTech vaccine causes more levels of IgG than IgA in saliva.
A total of 1,870 saliva samples and 1,829 serum samples were collected throughout the study. The saliva flow was normal for the majority of participants. However, patients with CLL or primary immunodeficiencies had lower saliva flow.
Differences in IgG responses in saliva
All patients showed signs of IgG antibodies in their saliva samples after the first dose of vaccine.
People living with HIV had a 12-fold IgG reactivity in saliva, and healthy patients had a 12-fold increase. After the second dose, people living with HIV had a 53-fold increase and healthy patients showed a 74-fold increase.
Over 90% of people living with HIV and healthy patients showed an IgG response in saliva samples 35 days after the first dose.
In patients who received allogeneic hematopoietic stem cell transplantation (HSCT) / chimeric T antigen receptor cell therapy (CAR-T), there was a moderate 3-fold increase in IgG reactivity in saliva 21 days after vaccination. However, after the second dose, IgG reactivity increased from 3 to 50 times, suggesting a robust immune response.
In people with chronic lymphocytic leukemia (CLL), organ transplants, and primary immunodeficiencies, poor humoral responses have been observed. These groups did not show detectable IgG levels in saliva until two weeks after the second vaccination. They also had lower anti-Spike-f and anti-S1 responses in saliva than people living with HIV or HSCT / CAR-T. The researchers suggest that the poor response after vaccination may be due to illness or immunosuppressants.
IgG response in saliva is associated with IgG antibody titers in blood serum
IgG reactivity in saliva samples was moderately associated with IgG levels 10 days after the first dose of vaccine. However, the correlation between IgG levels in saliva and serum strengthened after receiving the second dose and 2 weeks after the second dose.
In patients with CLL, HSCT / CAR-T, and primary immunodeficiency, IgG Spike-f or S1 reactivity in saliva samples was strongly associated with serum anti-S1 antibody titers 2 weeks after the second dose. .
People living with HIV have shown a moderate association between IgG levels in saliva and serum after full vaccination.
Being under the age of 60 was correlated with more potent SARS-CoV-2 specific IgG antibody titers.
Limitations of the study
The researchers have not integrated an antibody isotype analysis and their neutralizing power against SARS-CoV-2. Several variants of SARS-CoV-2, such as Delta, contain mutations in the spike protein that allow it to escape or weaken neutralizing antibodies.
Immunity against SARS-CoV-2 is not limited to neutralizing antibodies. Local memory B and T cell immunity plays an essential role against SARS-CoV-2. However, the presence of B and T cells in saliva samples was not explored in the present study.
Additionally, patients enrolled in the study were assessed for their humoral immunity after receiving the Pfizer-BioNTech vaccine, but how this behaves with other coronavirus vaccines remains unknown.
*Important Notice
medRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link