
[ad_1]
Hello. I am Dr. Kathleen Dooling, a doctor and shingles specialist at the CDC. I am here to talk to you about the new highly protective shingles vaccine and what we learned after registration with regard to reactogenicity. The recombinant shingles vaccine (brand name Shingrix) is a two-dose vaccine that protects more than 90% against shingles, even in the elderly.[1,2] CDC now recommends Shingrix as the preferred vaccine for herpes zoster in immunocompetent adults aged 50 years and older.[3]
I will start with a reminder of the recommendations for use and proper administration of Shingrix, which are very different from those of Zostavax, a recommended shingles vaccine since 2006. A table describing the differences between Shingrix and Zostavax is presented here. -Dessous.
The CDC recommends immunocompetent adults 50 years of age and older to receive two doses of Shingrix, the second dose being administered 2 to 6 months after the first dose.[3] Shingrix should be administered intramuscularly (eg in the deltoid region of the arm). This is very important because if Shingrix is administered subcutaneously, the patient is more likely to develop a reaction at the injection site with pain, redness or swelling, which happened to this patient, who received a subcutaneous dose of Shingrix over triceps. .

Figure 1. Pain, redness and swelling due to poorly administered Shingrix vaccination.
Local and systemic reactions to Shingrix are quite common. Shingrix clinical trials and reports to the Vaccine Adverse Event Reporting System (VAERS) provided a detailed picture of reactions to this vaccine. In eight clinical trials involving more than 10,000 people who wrote a reaction journal, 78% of those who received Shingrix reported at least one injection site pain, 38% redness, and 26% swelling.[1,2] For 1% to 3% of people, the area of redness and swelling exceeded 4 inches.[1,2]
In this figure, you can see the degree of redness that patients may feel a few days after receiving a properly administered dose of Shingrix.
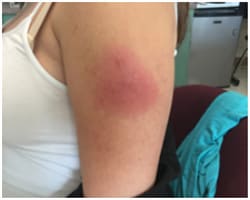
Figure 2 Redness even when Shingrix has been administered properly.
In addition to local reactions, approximately 1 in 10 people who received Shingrix in clinical trials reported systemic effects severe enough to limit activity, such as myalgia, fatigue, headache, chills, fever or gastrointestinal distress. intestinal.[1,2]
From October 2017 to June 2018, health care providers and patients submitted to VAERS just over 4,000 reports of events involving Shingrix.[4,5] Approximately 3.2 million doses of Shingrix were distributed during this period.[4] The most common symptoms reported at VAERS were fever, injection site pain, and erythema. In a rebaduring manner, the results of early Shingrix surveillance are consistent with the safety profile observed in clinical trials; serious side effects were rare and no unusual pattern was detected.[4,5]
Before administering Shingrix, the CDC recommends that you inform your patients of routine reactions to the vaccine. You must tell patients to expect reactions such as pain, swelling and redness at the injection site, as well as body pain, fever, and chills. Most reactions to Shingrix are spontaneously resolving and disappear within 2-3 days.[3]
You should encourage patients to complete the two-dose series of Shingrix, even if they have a reaction at the first dose. In clinical trials, a reaction at the first dose did not predict a reaction at the second dose. In addition, the effectiveness of a single dose of Shingrix has not been studied. If you do not have Shingrix in stock, you or your patients can visit the vaccinfinder.org website to locate it. Finally, report any clinically significant reactions to VAERS so that we can continue to monitor the safety of Shingrix.
For more information, visit the CDC Shingles Vaccine website. As a health care provider, your vaccination recommendations have the greatest impact on the decisions your patients make. Help protect your patients aged 50 years and older against shingles and its complications by strongly recommending vaccination against shingles. Thank you.
Table. Differences between Shingrix and Zostavax
Web Resources
Recommendations of the Advisory Committee on Immunization Practices for the Use of Herpes Zoster Vaccines
Information on Shingles Vaccine for Health Professionals
[ad_2]
Source link