
[ad_1]
Brrr … it's cold outside! Children flock to the television in the hope of hearing that there will be a snowy day; the bread and milk aisles of the grocery stores are empty because of an imminent snowstorm; and utility trucks spray salt or salt water on the roads.
We all know why the first two arrive. The kids are excited about a day off filled with chocolate and snowmen. Adults supply themselves with basic necessities. But what's new with these trucks?
They strive to protect slippery road users by spraying rock salt or salt water solution to prevent ice formation. This salt is very similar to the salt you have on your table: it is the same sodium chloride, NaCl. Some proprietary blends contain other salts, such as potbadium chloride (KCl) and magnesium chloride (MgCl), but they are not used as often as usual.
But it is an inexpensive and effective way to protect the ice roads with a simple scientific principle: to reduce the freezing point of solutions. The freezing point of pure water, the temperature at which it becomes ice, is 32 degrees Fahrenheit. So if there is snow, slush, or freezing rain and the soil is above 32 degrees Celsius, solid ice will form on the streets and sidewalks.
However, if the water is mixed with salt, the freezing temperature of the solution is below 32 ° F. The salt prevents the water molecules from forming solid ice crystals. The degree of depression of the freezing point depends on the salinity of the solution.
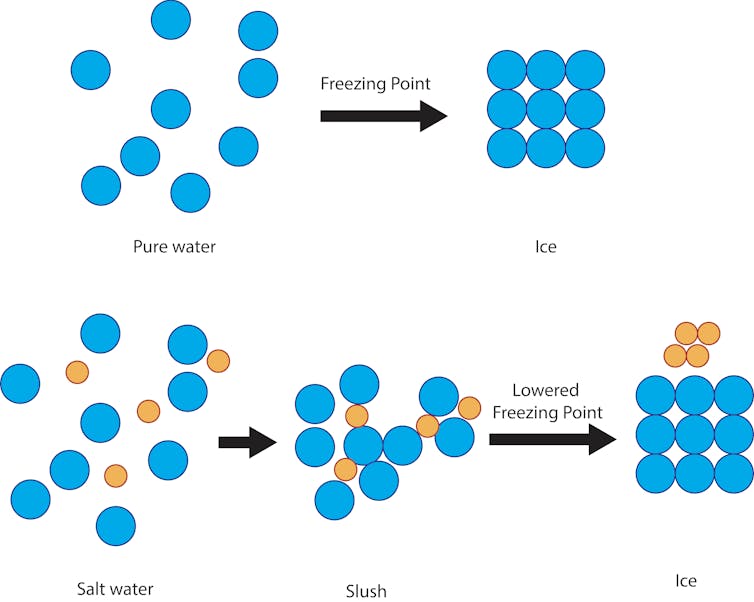 The salt prevents the water molecules from solidifying into ice crystals at 32 ° F, but remains slippery at this temperature, before finally freezing at about 15 ° F. Julie Pollock, CC BY-ND
The salt prevents the water molecules from solidifying into ice crystals at 32 ° F, but remains slippery at this temperature, before finally freezing at about 15 ° F. Julie Pollock, CC BY-ND
It is important to note that the salt must be in solution with liquid water for this principle to be respected. This is why many cities spray saline before ice forms.
The salt spilled on the ice depends on the sun or the friction of the car tires that cross it to melt the ice in melting snow that can mix with the salt and not be refrozen. Solid salt pretreatment depends on the warmer surface of the road to melt snow or freezing rain so that it can mix well with salt. This is also why pretreatment bridges – which are colder than other roads – usually do not work, and why you see signs "the bridge freezes before the road".
Another strategy used at these low temperatures is to put sand on the ice. Sand does not change the melting temperature, it just offers a rough surface to your tires to prevent slipping.
 A de-icing badtail of brine and beet juice, which could possibly work at temperatures as low as minus 25 ° F. Photo AP / Gene J. Puskar
A de-icing badtail of brine and beet juice, which could possibly work at temperatures as low as minus 25 ° F. Photo AP / Gene J. Puskar
Source link