[ad_1]
Hundreds of placebo-controlled NRT and Chantix studies are not blind as claimed
 In the face of a teenage ejaculatory and smoking epidemic, the US Food & Drug Administration (FDA) announced that it was reviewing "the potential role of drugs to help young people quit e-cigarettes "and invited the public to comment. In response, the comments below were submitted electronically to the FDA on 31 January 2019 (CTN: 1k3-97zu-27gy), with a printed copy posted on 1 February.
In the face of a teenage ejaculatory and smoking epidemic, the US Food & Drug Administration (FDA) announced that it was reviewing "the potential role of drugs to help young people quit e-cigarettes "and invited the public to comment. In response, the comments below were submitted electronically to the FDA on 31 January 2019 (CTN: 1k3-97zu-27gy), with a printed copy posted on 1 February.
From: John R. Polito, Nicotine Cessation Educator
Subject: Submission of comments to: Eliminate e-cigarettes among youth and others
Use of tobacco products: the role of pharmacotherapies; Public audience; Request
for comments: File ID: FDA-2018-N-3952, Agency: Food and Drug
Administration (FDA), parent agency: Department of Health and Human Resources
Services (HHS): Comment Tracking Number: 1k3-97zu-27gy
Title: How can we expect the FDA to be honest with juvenile drug addicts about smoking cessation when it has not yet been honest with smokers?
Commissioner Gottlieb and the FDA,
Although the following comments strongly criticize HHS and the FDA's history of reducing smoking cessation in the United States, I hope you read and think about it. Because if we refuse to consider where we started and where
At the present time, what hope is there to make the right decisions to best help the nicotine-dependent teenager?
Where are things
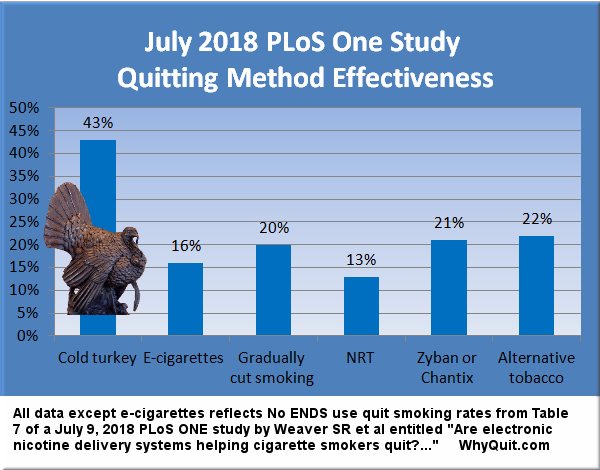
After 35 years of telling smokers that Pharma's nicotine is a drug and its therapeutic use, data from the most comprehensive study ever conducted on population-level smoking indicates that, as with all programs With nicotine withdrawal, the use of nicotine replacement can double your risk of relapse. and failure.[1] It is a statement of effectiveness in the real world that is reflected in previous studies.[2]
Yes, I understand the industry's argument that confusing smokers who have trouble quitting are more likely to search for and use approved products, while those who find it easier to quit do it less. But if it is correct, how can such confusion be greater than in clinical trials, as in the case of a study to recruit bait?
The results at the population level reflect the entire cessation. As GlaxoSmithKline and Pfizer, who stop marketing their products, never distinguish between levels of mind dependence it strikes, why population data
need adjustment?
Although the FDA focuses primarily on the regulation and approval of cessation products, section 918 of the Tobacco Prevention and Smoking in Families Act has also been specifically charged by the FDA with presenting to Congress a report on the best way to promote and encourage nicotine-free treatments, "In order to achieve" total abstinence from tobacco use. "
Despite the fact that 75 to 80% of ex-smokers stopped cold turkey,[3] the FDA has chosen to read section 918 in order to completely abolish the responsibility to investigate, understand, promote or encourage a sudden cessation of nicotine.
The FDA's response to the growing body of evidence that the product's approved clinical efficacy and actual effectiveness are diametric opposites has been to break the head in the sand.
Now, the FDA seems ready to encourage Nicorette, Nicoderm CQ, Habitrol and Zyban among young addicts dependent on the electronic cigarette.
What is needed is other studies such as Weaver 2018 and Doran 2005,[1] studies
provide a complete and complete picture of the productivity of population-level dropout methods
and images of efficiency. And not just for adults but for teens
as well.
Unfortunately, this will not happen. Why so negative? Because I lost hope. Because I saw the US Department of Health and Human Services (HHS) keep hidden the only known investigation into the stop methods produced by the government (Hartman 2006 NCI). In US PATHS version 944, page 2018, there is no question of suddenly stopping nicotine, cold turkey, or quitting without help, while posing multiple questions about each approved product.
As difficult as it may seem, HHS continues to deceive smokers by telling them that "few people" are able to leave turkey bluntly (Clearing the Air, 2006, NCI – see pamphlet on page 10). Because, year after year, HHS intentionally keeps
smokers, smokers and teenagers in the dark on the most important issue of quitting smoking: how the vast majority of smokers manage to end their chemical dependence on nicotine and become old users at ease (see thousands and thousands of documents "smokers" of the HHS).
Why would HHS oppose the creation and sharing of comprehensive, factual and robust photos on nicotine, tobacco and smoking in the United States, demonstrating what is effective and productive and what is not? A number of reasons.
The most obvious is the risk that the truth will weaken the FDA's mediation of stopping smoking, tobacco and nicotine.
The FDA, CDC and NCIs know since the Pierce 2002 JAMA study that they have fully invested their credibility and reputation in nicotine pharma, and that "since they are available over the counter, NRTs do not seem more effective for increasing long-term revenues. stop successfully in California smokers. "
Instead of laying the shovel, or after Mooney 2004, launching an investigation to find out if it was ever possible to blind the experienced poor as to the recognition of the presence or absence of their Withdrawal syndrome, the FDA, the CDC and the NCI continued to dig.
How we got here
What we need is that we have been waiting for a long time for the FDA to be alerted to serious concerns
June 22, 1983, before approving the nicotine gum on January 13, 1984, and since then knowingly helped to conceal it.
As this review shows, although placebo is the gold standard in most research, it has been allowed to fly as part of the fight against smoking.
Asked to apply the FDA's safe and effective standards to a natural insecticide, the main reason why the Family Smoking and Tobacco Control Act of 2009 (FSPTCA) was granted to the FDA and not to The Federal Trade Commission's jurisdiction over tobacco and nicotine was born 25 years ago. June 22, 1983, the
FDA Advisory Committee on Drug Abuse Mistaken in Declaring Adequate and Well-Controlled Studies Show Efficacy of Nicotine Gum Increasing Chances of Quitting Counseled Participants .
The Advisory Committee asked the most critical questions Wednesday morning in June. What he did not think about was that before being effectively judged for Nicorette to be effective, his concerns remained unanswered.
Nicotine Psychoactive
Dr. Jasinski discussed with the Committee that nicotine is a psychoactive drug. "I define it when given against placebo in various circumstances [it has] the ability to change mood, emotional states, thinking,
and perception. " [Page 98]
"Delivered intravenously or by inhalation, it is psychoactive and physiologically active. that is, people can distinguish it from placebo and that they can discriminate the contents of cigarettes when you smoke them in certain characteristic ways. They can also differentiate the different contents of nicotine cigarettes. They can also distinguish bolus saline and various doses of nicotine. intravenously. " [Page 98]
Dr. Jasinski went on to say that the nicotine gum "is psychoactive. Subjects can discriminate. [Page 105].
At that time, the committee openly declaring that the active group knew that they were receiving nicotine, while the placebo group could feel it, and no evidence to the contrary, all studies reviewed should have been declared non-blind. compromised by the awareness of the badignment, the measurement of expectations, inflated or diminished by the recognized value of the board or the importance of contacts during studies, and by a motion to adjourn. But that did not happen.
Previously, the committee had sampled the placebo gum used in the Christen dental study. [
Page 48]
Placebos "Nasty"
"Another of my concerns was that neither the protocol nor the study report submitted by the sponsor indicated there was a discernible difference between Nicorette's taste and placebo chewing gum" said Dr. Marticello. "It's a factor that could have affected the blindness of the study. I guess some people have already tasted the placebo, but not Nicorette. " [Page 87]
"Are placebos the same as nicotine gums containing 2 milligrams? asked Dr. Goodwin earlier. "Can you tell the difference between them and the active drug?" [Page 30]
"I have not chewed this placebo before, and it's very different," said Dr. Jones. "I find that not unpleasant." [Page 30 ]
What about the active group when chewing chewing gum 2 mg?
Dr. Jones had already sampled the nicotine gum. He stated that he had "a different taste, and that you have effects – I would call it side effects – you get the effects of nicotine, at least me, as a non-smoker" . [Page 30]
"I'm pretty sure the person can detect the pharmacological effects," said Dr. Martz. "But the taste is – – we put as much nastiness as we could get." [Page 30]
According to studies showing how each gum, patch or pellet was active, they were generally provided by the pharmaceutical industry.
More than 200 placebo-controlled TRN trials and, over the course of the studies, the industry better understood which placebo formulations were generating interesting results or not.
Active Placebos
Reese T. Jones, PhD, was concerned that the other main study submitted in support of Nicorette's approval, the
Russell study, had used an active placebo containing nicotine.
It has been explained that, although the active gum is a 2 mg end-use product, "the placebo was 1 milligram unbuffered gum and the lack of buffer meant that even the lowest dose of nicotine was much less well absorbed" . [Page 42]
"How much nicotine should be in it?" He asked. "And since we do not know the bioavailability at this dose in unbuffered form, really, I think Dr. Russell's data is rebaduring, but it's certainly not the kind of data I would ask if it was almost possible. any other drug, treating almost anything other than tobacco addiction. " [Page 106]
Think about what Dr. Jones said. Because it's about smokers and patients already addicted to nicotine, science can be sloppy, it's permissible to guess, and we do not need to know the exact consequences of adding 1 mg of unbuffered nicotine to placebos.
This is important because the use of active placebos has also been recognized in several studies on nicotine patches (1996, 1997, 2002).
Have active placebos been fortified with just enough nicotine to keep users in the trap and weaning jets: not providing enough to satisfy cravings, or allowing them to clean themselves up, start re-sensitizing and Go beyond
maximum withdrawal within 3 days?
To what extent was the use of active placebos widespread? We have no idea. It would seem that after hearing all of this, the FDA does not care, because there has been no known study, no investigation and no claim of use by the industry has not been presented.
The question of badessing blinding integrity without response was asked by Dr. Paul. "To what extent did your patients … predict whether they were on placebo, how accurately could they predict whether they were on placebo or did you do these experimental experiments?" Because the differences, although significant, perhaps twice in terms of abstinence, are still relatively small, and I'm really concerned that these are real differences between drugs and placebo, between the differences between the drugs and the inactive placebo and the active placebo, and I am curious to know. if patients could say retrospectively and reliably. " [Page 61]
Dr. Russell's answer? "We did not actually inform the patients that they were getting the placebo." "We said we were trying on nicotine. Would they like to participate in a clinical trial of chewing gum containing nicotine? " [Page 61]
What was totally lacking in the committee was the fact that experienced people had become experts in recognition of their withdrawal syndrome and that these discriminating experts would be fully aware of their mission within 24 to 48 hours of stopping. (maximum weaning).
Before the vote in committee, Dr. Leber said, "If you were to conclude that [nicotine gum] It is a horror of public health, so say it and tell us not to continue, because we do not want to make any mistakes. " [Page 113]
In response, Dr. Paul, like Dr. Jones, wanted to know if there was any data on the 1.2 million people who had used nicotine gum in countries that had already approved it. [Page 114]
The Nicorette manufacturer has not yet provided the FDA Advisory Committee with basic information on population effectiveness, critical performance data that has been hidden every year since.
The tests NRT, Zyban and Chantix are not blind
A series of studies by KA Perkins teaches us that smokers and nicotine-naive participants can be trained and conditioned to reliably distinguish varying doses of nicotine.
Mooney 1984 examined the few clinical trials that led to blinding integrity badessments of very poor quality. She found that placebo-controlled clinical trials were generally not blind, contrary to what was claimed in the sense that participants could correctly report their badignment at a rate well above random.
Mooney concluded by encouraging researchers to conduct quality integrity badessments and adjustments as needed and cautioning against the consequences of failure to meet this obligation.
"To determine the prevalence of failure, clinical trials on NRTs must uniformly test the integrity of the blinds in the study. In addition, if failure of blindness is observed, further efforts should be made to determine whether failure of blindness is related to the results of the study and, if so, to provide an estimate of treatment outcomes. adjusted for blindness bias. Without these methods and badyzes, the validity of clinical trial results on NRTs could be questioned. "
Surprisingly, only two post-Mooney studies included blinding integrity badessments and only one made adjustments. In Dar 2005, the fact that 3.3 times more placebo users were able to correctly identify their randomized badignment as declared erroneous modified the outcome of the study.
In Rose 2009, less than a week after quitting, 4 times more placebo patch users were able to correctly report their badignment as erroneous ("out of 165 subjects receiving placebo patches, 27 believed they had received active patches, 112 thought they had not been, and 26 were not sure ").
If more than 50 million people "double your chances" have tried to stop using nicotine gum in the United States since 1984, what are the successes? After 29 years as the cornerstone of the FDA's smoking cessation and billions of dollars spent on marketing, a Gallup poll in July 2013 found that only 1% of people who quit in the United States were successful. with nicotine gum.
According to the 2013 Gallup Poll, 92% of successful ex-smokers said they did not credit NRT, Zyban or Chantix. Almost all had succeeded without her.
Unfortunately, it seems that the correct answer to the public health horror question posed by Dr. Leber's nicotine gum is categorical: "yes, it's true". Under real-life conditions, as almost all members of the population suggest
By abandoning the method data, OTC NRT compromises the success of the shutdown. At what cost in terms of late cessation and lives lost?
FDA lets marketing go wild
According to Dr. Jones, is it a coincidence that the last concern presented to the FDA Advisory Committee in 1983 responds to what I have been trying to bring HHS attention to for almost two decades, allowing the tail to lie while stirring the dog. Double your chances of leaving product marketing at Pfizer's new Chantix
Campaign "Keep on smoking" and "I'm a tough guy, Ray and I could not leave cold turkey", marketing has been allowed to hit, undermine and undermine the confidence in the production and people of his country , unbeaten champion of efficiency, cold turkey.
As Dr. Jones said, "I guess most smokers who want to quit quit smoking without any special work intervention." I saw good data on this and maybe Dr. Russell or someone else might have data on how many smokers are able to quit without any intervention. "
"What the availability of this will make, the gum will do, is more prone to resort to pharmacotherapy when there is a chance that they do not need pharmacotherapy." "[I]t is a consideration in terms of maybe require
the best proof of efficiency we can ". [Pages 122-123]
Pharmaceutical Influence Controlling US Termination Policy and HHS
What Dr. Jones could not have predicted was that the quitting pharmaceutical industry would create a pool of qualified and well-paid senior investigators, some of whom would allow HHS to play a major role in the development of the US official policy on cessation.
What the committee could not have known then was that seventeen years later, in June 2000, under the direction of a president who sat in a million-dollar chair with Glaxo-Wellcome (the Nicorette manufacturer), 11 of the 18 panel members declared financial ties from the pharmaceutical industry, HHS support for the method that had produced almost all successful US ex-smokers was about to be permanently banned .
How could Dr. Jones anticipate that, similar to Glaxo-Wellcome's written agreement on the endowment chair, the US 2000 guidelines would now require all smoking patients to quit smoking using drug therapy ( compare recommendation 7 of the guidelines on page iv)?
Today, the pharmaceutical industry pays 75% of the FDA's drug approval budget, while the FDA tobacco program is 100% funded by the rights to use tobacco products, including 96, 33% are paid indirectly by chemically enslaved cigarette smokers.
Both smokers and teenagers deserve the truth. But respectfully, that did not happen with
Mitch Zeller as director of the Tobacco Bureau of the FDA since March 2013.
Should we close the spirits on the fact that GlaxoSmithKline would have everything to gain from recommending the use of TRNs by addicted teenagers, or that between 2002 and 2013, Zeller was senior vice president of Pinney Associates, the company marketing GlaxoSmithKline's exclusive smoking cessation products?
The FDA's September 2012 hearing with Zeller's FDA management, its badysis of the "end-point tobacco" risk continuum, does not mention the re-establishment of nicotine addiction. Instead, much to the delight of GlaxoSmithKline, Zeller's ultimate goal is the long-term use of "the current generation of nicotine-based medicinal products such as gum, patches and lozenges".
Ask yourself if the phrase "your odds" suggests that we refer to actual efficacy results or to clinical trial efficacy wins over placebo users who wanted get what they actually had but did not get it.
If the first, a December 2017 FDA page created under Zeller's supervision, openly feeds smokers and teens into believing that "the use of FDA-approved discontinuance drugs can double your chances of give up smoking.
Regarding the final thought of Zeller's late-stage TRN, the FDA's new page also tells smokers that "the FDA recognizes that some people may need to use these products longer to stay smoke-free. Talk to your health care provider to determine the best treatment for you. "
Now, Pharma wants our children too
Really too friendly and confident about pharmaceuticals, a NRT-approved house of cards, built on uncomfortable placebos that did not diminish the recognition of maximum withdrawal, or the ability to tease and torture the control group. Relapse, The FDA stands ready to extend to children and adolescents the most murderous science sham in history.
I beg the FDA to resist the urge.
What our nicotine kids need is honest, accurate recovery information, not more nicotine.
They need to hear their health officials tell them the truth about nicotine and addiction, that they might begin to lose the autonomy to turn around and get away from it afterwards. Be vaping just a moment or two.
Children and adolescents need to understand what nicotine addiction is and want to say that, like them, they feel cravings to eat two or three times a day, that the same pathways of dopamine in the brain can quickly begin to behave as if nicotine is a food.
Ask students to try to imagine their brain generating nicotine cravings 5, 10, 15 or even 20 times a day, every day, from waking to bed, for the rest of their days. Ask them to imagine difficulties in focusing in clbad because there is a war in their brains while they are trying to fight against impulses.
Students need to understand that nicotine addiction is a REAL drug addiction, a mental desire disorder and a real mental illness as real and permanent as alcoholism.
If they can completely and comfortably stop their chemical dependence, they can neither kill nor cure it. After having quit smoking, all it takes is a breath, an immersion, a vape or a chewing and they will soon realize that their brain is missing, plotting to
get or even beg for more.
If the FDA really wants to promote the successful abandonment of teens, it needs a correct and accurate answer to the most basic question in the field. What is the key to the success of an abrupt cessation of nicotine?
I submit that the answer is the exact opposite of relapse-relapse counseling currently shared by HHS at SmokeFree.gov.
Imagine the folly of teaching convalescent alcoholics that "slip and have a [drink] or even go back to [drinking] for a little time do not miss. It's normal. "" A slip does not make you [drinker] again."
Instead of inviting, encouraging and promoting relapses, teens and nicotine-dependent adults need to be aware that only one breath and up to half of their dopamine pathways in the brain would be occupied. by nicotine.
As the 1990 Brandon study reveals, almost everyone who has "tasted" a cigarette has relapsed (88%). "The high rate of return to regular smoking once a cigarette has been tasted suggests that the distinction between an initial failure and a total relapse may be unnecessary."
The 1992 Garvey study followed 235 adult adult smokers for a full year after attempting to quit. He found that "those who have smoked cigarettes at all in the
Post-cessation period (that is, expired) had a 95% probability of resuming their usual pattern of smoking eventually. "
All the articles and videos of Joel Spitzer, the most educated nicotine cessation educator in the US, end in the same way. Joel reminds them of the rule that, if followed, provides 100% chance of success, never take another puff.
Joel's library houses nearly 500 video lessons and over a hundred articles on almost every topic related to the brutal cessation of nicotine imaginable. To the extent that HHS has never had difficulty in mentioning products approved for smoking cessation by brand (as it is the case at the bottom of the invitation to comment), in a spirit positive action, I encourage the FDA to start referring to the Joel Library for those looking for abrupt information. cessation of nicotine.
In conclusion, the FDA is almost as entrapped and dependent on the discovery of placebo-controlled smoking cessation products as any student at the time of his election. There are two options. He can continue to dig or finally demand complete and complete images of the real world, while initiating his own inquiry into its integrity.
I sincerely hope that he chooses the latter.
Respectfully,
John R. Polito, JD
Nicotine cessation educator
References:
[1] Tisserand SR, Huang J, Pechacek TF, JW Heath, Ashley DL, MP Eriksen. Do electronic nicotine delivery systems help smokers quit? Results of a prospective cohort study conducted among American adult smokers, 2015-2016. 2018 PLoS ONE 13 (7): e0198047.https://www.ncbi.nlm.nih.gov/pubmed/29985948.
[2] Doran CM, Valenti L, Robinson M, Britt H, Mattick RP. Tobacco status of Australian patients in general practice and attempts to stop smoking. Addict Behav. May 2006; 31 (5): 758-66. Epub 2005 31 August.
https://www.ncbi.nlm.nih.gov/pubmed/16137834
; See also Twyman L, Bonevski B, Paul C, Bryant, West, Siahpush, M, D, Estee, Oldmeadow, Palazzi, K. What are the factors badociated with abstinence among socio-economically disadvantaged smokers? A
cross-sectional survey on the use of smoking cessation aids and the approach to smoking cessation. Drug Alcohol Rev. 2018 Feb; 37 (2): 170-179. doi: 10.1111 / dar.12561. Epub 2017 14th of June. Https://www.ncbi.nlm.nih.gov/pubmed/28616900
; and Ferguson J, Bauld L, J Chesterman and Judge K. English smoking treatment services: results over one year. Addiction 100 (Suppl.2), 59-69 2005, see Table 6 https://whyquit.com/studies/2005_uk_nhs_1_year_ferguson.pdf
https://academic.oup.com/aje/article/187/11/2397/5046037Tarik https://www.ncbi.nlm.nih.gov/pubmed/29955810; voir aussi Doran CM, Valenti L, Robinson M, Britt H, Mattick RP. Statut tabagique des patients australiens en médecine générale et tentatives d’arrêt du tabac. Addict Behav. 2006
May; 31 (5): 758-66. Epub 2005 31 août. Https://www.ncbi.nlm.nih.gov/pubmed/16137834.
Moi, John R. Polito, je suis entièrement et seul responsable du contenu de cet article. Toute erreur factuelle sera corrigée rapidement après notification par courriel à [email protected].
Comment arrêter de fumer
Regardez près de 500 vidéos supplémentaires pour arrêter de fumer
Nos livres électroniques gratuits
<img src = "https://whyquit.com/NTAPcover.jpg" width = "137" height = "174" border = "3″ alt=”Cliquez pour en savoir plus sur Never Take Another Puff, un livre PDF gratuit pour arrêter de fumer”/>
Lisez les deux et détruisez les angoisses causées par la peur!

Découvrez la Turquie intelligente
- WhyQuit.com – WhyQuit est le plus ancien forum Internet consacré à l'art, à la science et à la psychologie du sevrage brutal, méthode d'arrêt du tabagisme utilisée par la vaste majorité de tous les ex-fumeurs à long terme qui ont réussi.
- Dépendance à la nicotine 101 – Guide de base de WhyQuit pour comprendre la dépendance à la nicotine.
- Index de sujet de cessation de nicotine – Un index alphabétique de plus d'un millier d'articles, de vidéos et de discussions de groupe sur la cessation de la nicotine.
- Joel's Library – Joel Spitzer a commencé à présenter des cliniques et des séminaires pour arrêter de fumer en 1976. Directeur de l'éducation de WhyQuit depuis 2000, la bibliothèque de Joel abrite le travail de sa vie. Il comprend le "Guide de la cesser de fumer quotidien" de Joel, plus de 100 articles originaux sur l'arrêt du tabac, son ebook gratuit "Never Take Another Puff" et sa collection sans cesse croissante de plus de 400 vidéos contre le tabagisme.
- "Freedom from Nicotine – Le voyage de retour" – Rédigé par John R. Polito, ancien fumeur de 30 ans et fondateur de WhyQuit en 1999, Freedom from Nicotine partage les connaissances scientifiques sous-jacentes à la dépendance à la nicotine et à l’arrêt brutal de la nicotine.
- Turkeyville – Imagine t'entourer de plus de 10 000 démolisseurs de dinde froide. Turkeyville est un groupe de soutien Facebook réservé exclusivement aux démissionnaires de régime.
- Liberté – Freedom était le groupe de soutien pour l’arrêt du tabagisme originel de WhyQuit en 1999. N'acceptant plus de membres, ses 453 000 publications archivées continuent de partager des informations sur la récupération.
La connaissance est une méthode d'abandon
Source link