[ad_1]
For the first time ever, researchers have successfully transformed human stem cells into mature insulin-producing cells, a breakthrough in efforts to develop a cure for type 1 diabetes (T1).
Replacing those cells, which are lost in patients with T1 diabetes, has long been a dream of regenerative medicine, but until now scientists have not been able to determine how to produce cells. in a lab dish that work as in healthy adults. .
"We can now generate insulin-producing cells that look and act much like the beta cells in the pancreas that you and I have in our body. This is a crucial step towards our goal of creating cells that could be transplanted into diabetic patients, "said Matthias Hebrok, director of UCSF Diabetes Center and lead author of the new study, published in Nature Cell Biology.
T1 diabetes is an autoimmune disease that destroys insulin-producing pancreatic beta cells, usually in childhood. Without insulin's ability to regulate glucose levels in the blood, peaks in blood sugar can severely damage organs and eventually lead to death. This condition can be managed by regularly taking insulin injections during meals, but people with type 1 diabetes still suffer serious health consequences, such as kidney failure, heart disease and a stroke.
RELATED: An 8-year study shows that a simple treatment can reverse type 1 diabetes to almost undetectable levels
Patients facing potentially life-threatening complications of their disease may be eligible for a pancreas transplant from a deceased donor, but they are rare and waiting time is long: about 1.5 million people living with type 1 diabetes in the United States, about 1,000 get pancreas transplants each year. The procedure is also risky: recipients have to take immunosuppressive drugs for life and many of the transplants fail for one reason or another. Transplants of only pancreatic "islets" – clusters of cells containing healthy beta cells – are currently undergoing clinical trials, but still rely on pancreas from deceased donors.
That's why Hebrok and other diabetes researchers have long hoped to use stem cells to grow healthy beta cells in a lab, so they can be transplanted into patients without waiting for pancreas transplants. Islets – but for years scientists have been unable to understand how to program stem cells into fully mature beta cells.
"The cells we and others produced were stuck in an immature stage where they were unable to respond properly to blood glucose levels and to secrete insulin properly. This has been a major bottleneck for the industry, "said Hebrok.
MORE: Seven years after experimental treatment for MS, woman still has no symptoms
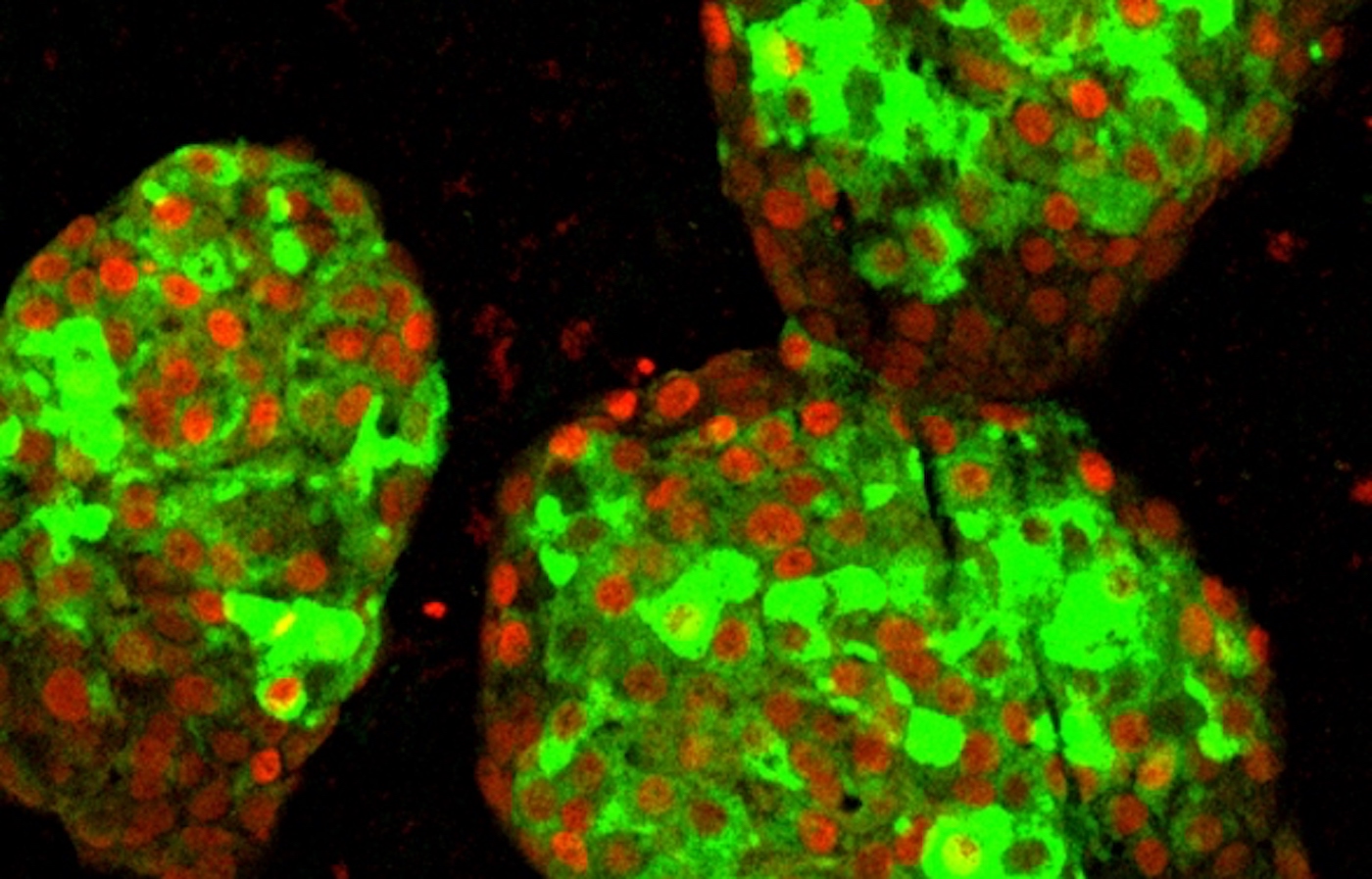
In this new study, Hebrok and his colleagues, led by Gopika Nair, a postdoctoral fellow, realized that the key to growing fully mature beta cells in the lab was a neglected facet of beta cell development – the physical process by where the cells separated from the rest of the pancreas and formed the so-called islets of Langerhans.
"A key principle in biology is that form follows function, so we decided that islet formation could be an important process for beta cells to mature properly," Nair said.
When the researchers duplicated this process in laboratory cans by artificially separating partially differentiated pancreatic stem cells and reforming them into clusters resembling islets, cell development suddenly progressed. Not only did beta cells begin to react to blood sugar levels, more like mature insulin-producing cells, but the entire "neighborhood" of the islet – including alpha and delta cells less well understood – also seemed to develop in a way never seen before. laboratory setting.
LOOK: A boy supposed to be non-verbal can speak after the dentist has discovered that he was simply "attached to the tongue"
The researchers then transplanted these lab-grown "islets" into healthy mice and found that they were working in just a few days – producing insulin in response to blood glucose, much like the islets of the liver. 'animal.
In collaboration with bioengineers, geneticists and other UCSF colleagues, the Hebrok team is already working to move regenerative therapies from dream to reality, for example by using genetic modification. CRISPR to make these cells transplantable without the need for immunosuppressive drugs, or by looking for drugs that could restore islet function in patients with T1 diabetes by protecting and developing their remaining few beta cells to revive the production of diabetes. pancreatic insulin.
"Current therapies such as insulin injections only treat the symptoms of the disease," Nair said. "Our work indicates several exciting ways to finally find a cure."
"We are finally able to move forward on several fronts that were previously closed to us," Hebrok added. "The possibilities seem endless."
(Source: University of California at San Francisco)
Heal your friends from negativity by sharing the good news to social media – Photo by Hebrok Lab / UCSF
[ad_2]
Source link