[ad_1]
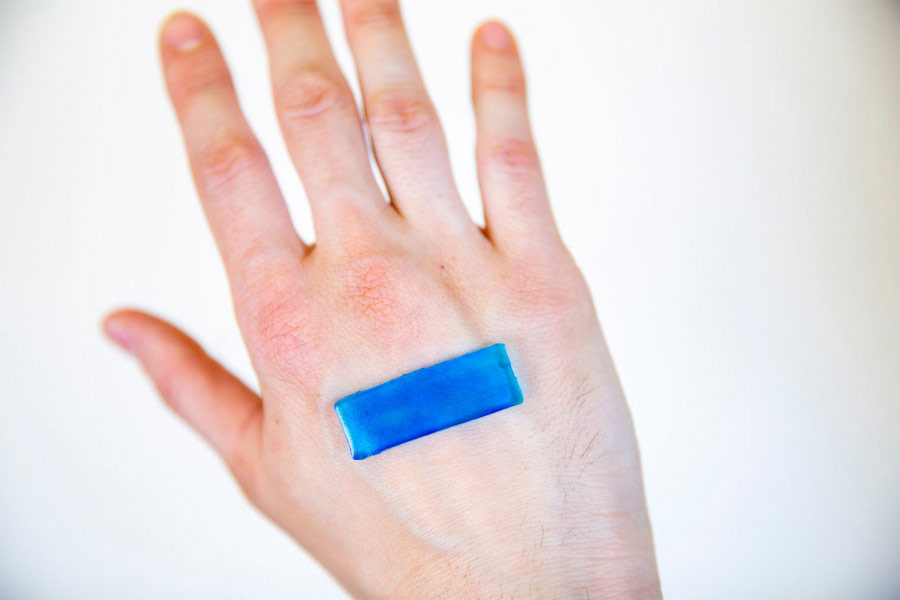
The active adhesive bandage contracts when it reaches body temperature, which allows it to accelerate the healing of open wounds on the skin. Credit: Wyss Institute of Harvard University
Cuts, scrapes, blisters, burns, splinters and punctures – our skin can be broken in many ways. Most treatments for skin wounds simply involve covering them with a barrier (usually an adhesive gauze dressing) to keep them moist, limit pain and reduce exposure to infectious microbes, but they do not facilitate actively the healing process.
More sophisticated dressings capable of monitoring aspects of wound healing such as pH and temperature and administering wound site therapies have been developed in recent years, but their manufacture is complex, expensive and difficult to personalize. which limits their potential for widespread use.
Today, a new evolutionary approach to accelerate wound healing has been developed, based on heat-sensitive, mechanically active, extensible, resistant, highly adhesive and antimicrobial active hydrogels: active adhesive active ingredients (AAD). Created by researchers at the Wyss Institute for Biologically Inspired Engineering from Harvard University, the Harvard School of Engineering and Applied Sciences John A. Paulson (SEAS) and the McGill University, DAAs can close wounds much faster than other methods and prevent bacterial growth need additional devices or stimuli. The research is reported in Science Advances.
"This technology could be used not only for skin lesions, but also for chronic wounds such as diabetic ulcers and pressure ulcers, for the administration of drugs and as components of therapies based on soft robotics," he said. Corresponding author David Mooney, Founder, Senior Fellow of the Wyss Institute and Professor of Bioengineering of the Robert P. Pinkas Family at SEAS.
DAAs are inspired by developing embryos, whose skin is able to heal completely without the formation of scar tissue. To achieve this, embryonic skin cells around a wound produce fibers consisting of actin protein that contract to bring the edges of the wound together, such as a closed cord bag. Skin cells lose this ability once the fetus has pbaded a certain age and any injury that occurs after that moment causes inflammation and scarring during the healing process.
To replicate the contractile forces that result in the closure of embryonic wounds, researchers have expanded the design of resistant adhesive hydrogels developed previously by adding a heat-resistant polymer called PNIPAm, which repels water and shrinks to about 90 degrees Fahrenheit. The resulting hybrid hydrogel begins to contract when it is exposed to body heat and transmits the force of the PNIPAm contraction component to the underlying tissue bonds of the underlying tissue between the hydrogel d & # 39; 39, alginate and tissue. In addition, silver nanoparticles are incorporated into the DAA to provide antimicrobial protection.
"The ADA is bound to the pig skin with an adhesive strength greater than 10 times that of a bandage and prevented the proliferation of bacteria, so this technology is already much better than the the most commonly used wound protection products, even before taking into account its wound closure properties, "said Benjamin Freedman, a postdoctoral fellow at the Mooney Laboratory of the Graduate School of Arts and Sciences, who is leading the project.
To test the quality of their closed wounds, the researchers tested it on mouse skin patches and found that it reduced the wound size by about 45 percent compared to the almost no modification of the zone in untreated samples and wounds closed more rapidly. treatments including microgels, chitosan, gelatin and other types of hydrogels. DAA also did not cause inflammation or immune response, which indicates that it is safe for use in and on living tissues.
In addition, researchers were able to adjust the degree of wound closure performed by DAA by adding different amounts of acrylamide monomers during the manufacturing process. "This property could be useful when applying adhesive on wounds on a joint such as the elbow, which moves a lot and would probably benefit from a more flexible bond, compared to a more static area of the body like the shin, "said co first author, Jianyu Li, a former postdoctoral fellow at the Wyss Institute, who is now an adjunct professor at McGill University.
The team also created a computer simulation of AAD-badisted wound closure, which predicts that the skin could shrink at a rate comparable to that of mouse skin, which would indicate a higher probability of presenting a benefit. clinic in human patients.
"We continue this research with studies to learn more about the impact of mechanical signals exerted by ADA on the biological process of wound healing and on the operation of ADA at different temperatures." , body temperature may vary in different places, "said Freedman. . "We hope to pursue further preclinical studies to demonstrate the potential of AAD as a medical product, then work on marketing."
Other authors of the article include Serena Blacklow, a co-first author, a former Mooney lab member who is now a graduate student at the University of California at San Francisco; Mahdi Zeidi, graduate student at the University of Toronto; and Chao Chen, a former graduate student of SEAS who is now a postdoctoral fellow at UMbad Amherst.
This research was funded by the National Institutes of Health, the Wyss Institute for Biological Inspiration Engineering at Harvard University, the Natural Sciences and Engineering Research Council of Canada. Canada, the Canadian Foundation for Innovation and the Harvard University Center for Materials Research and Engineering.
[ad_2]
Source link