[ad_1]
Despite the advent of numerous vaccines and the discovery of passive monoclonal antibodies for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), there remains an urgent need for definitive therapies for patients with coronavirus disease. -2019 (COVID-19).
Current global challenges in the fight against the pandemic include – the emergence of viral variants; decline in immunity after vaccination; vulnerability of immunocompromised people to severe symptoms even after vaccination; and chronic or persistent infection which generates variants which may elude immunity.
Anti-inflammatory drugs have little clinical benefit in certain subgroups of patients. Therefore, the control and eradication of this viral spread is the need of the hour.
Mupadolimab is a humanized IgG1κ FcγR anti-CD73 monoclonal antibody (mAb) that activates CD73POS B cells. Mupadolimab is known to increase the expression of markers associated with B cell maturation and antigen presentation and increase the secretion of immunoglobulin M (IgM) and immunoglobulin G (IgG), in vitro.
Researchers at various institutions in the United States have undertaken a study and examined the use of mupadolimab to stimulate antiviral immune responses and improve clinical outcomes in COVID-19. The study is posted on the medRxiv* server pending peer review.
ddd
This was an open-label phase 1 trial with mupadolimab in hospitalized COVID-19 patients, the results of which led to the launch of a randomized, double-blind, placebo-controlled phase 3 study. However, the latter was terminated in early mid-2021 due to the drop in the number of COVID-19 cases in the United States.

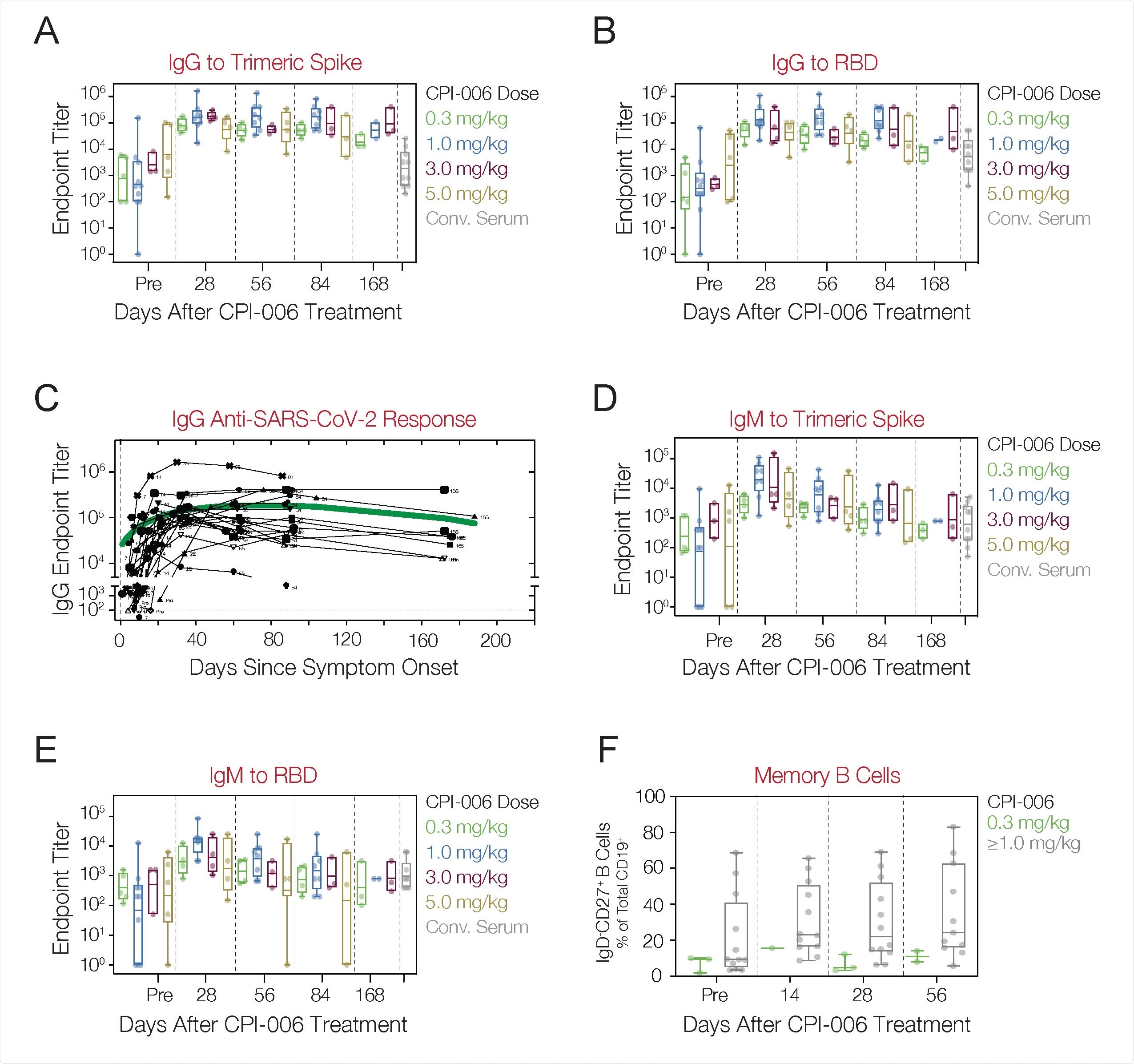
Anti-SARS-CoV-2 antibodies and cellular responses in COVID-19 patients treated with mupadolimab. A, B) Patients received a single dose of 0.3, 1.0, 3.0, or 5.0 mg / kg mupadolimab and endpoint IgG titers at TS (A) and RBD (B ) were measured at pretreatment and on days 28, 56, 84, and 168. C) Longitudinal analyzes of IgG against SARS-CoV-2 in the subject treated with mupadolimab. The green line is a spline fit fit. Each black line represents an individual patient. D, E) Patients received a single dose of 0.3, 1.0, 3.0, or 5.0 mg / kg mupadolimab and endpoint IgM titers at TS (D) and RBD (E ) were measured at pre-treatment and on days 28, 56, 84, and 168. F) Frequency of circulating memory B cells (CD19POSIgDNEGCD27POS) in the CD19POS gate at baseline and after treatment in patients treated with 0.3 mg / kg mupadolimab compared to ≥ 1.0 mg / kg. The data are presented as a box-whisker plot with a geometric mean and an interquartile range. Each point represents a patient. Also shown are the titers of convalescent patient serum obtained 4 to 6 weeks after POS.
Here, an animal model was used to determine whether mupadolimab could enhance antigen-specific immune responses to SARS-CoV-2. Mice vaccinated with trimeric spike protein (TS) plus mupadolimab were observed to produce antigen-specific anti-TS human antibodies. In contrast, those receiving TS plus isotypic control did not elicit a response. These antibody responses were antigen specific.
The results indicated that mupadolimab induces antigen-specific humoral immunity – to immunize the TS protein – and has the potential to elicit antibody responses to SARS-CoV-2 in patients with COVID-19.
COVID-19 Immunotherapy – Open Phase 1 Trial
The escalating single-dose phase 1 open-label trial evaluated safety, immunologic effects, and clinical outcomes in hospitalized patients with mild to moderate COVID-19. Cohorts of patients received intravenous infusions of 0.3 mg / kg, 1.0 mg / kg, 2.0 mg / kg, 3.0 mg / kg or 5.0 mg / kg of mupadolimab, administered in 5 10 minutes away.
The time interval from symptom onset (POS) to administration of mupadolimab was found to be 1 to 21 days, with a median of 8 days. No drug-related adverse events or changes in quantitative serum immunoglobulins have been reported. All patients recovered with improvement in inflammatory markers and symptoms and were discharged after a median of 3 days. No patient required invasive or non-invasive mechanical ventilation.
Immune Responses in COVID-19 Patients Treated with Mupadolimab
IgG antibody titers against SARS-CoV-2 TS and / or receptor binding domain (RBD) were markedly increased in all patients 28 days after a single infusion of low doses of mupadolimab. At the same time, lower antibody titers were recorded in the cohort receiving 0.3 mg / kg. Serum mupadolimab concentrations reached levels greater than 1 µg / ml for more than 24 hours at doses of 1 mg / kg and above – concentrations known to activate B cells in vitro. In addition, IgG titers were maintained without depletion for up to six months after symptom onset.
In addition, antibody titers were higher with this therapy than with convalescent sera from cured patients. Anti-SARS-CoV-2 IgM titers showed a similar trend with decreasing titers seen beyond 84 days.
Preliminary evidence suggests that mupadolimab increases the frequency of memory B cells. However, memory B cells did not increase in the lower dose group.
Sustained and high neutralizing titers have been observed in patients following treatment with mupadolimab – with DI50 values up to 24,000 which persisted for more than 56 days after symptom onset. In addition, day 28 serum from patients treated with mupadolimab effectively neutralized the B.1.1.7 variant.
The results suggest that treatment with mupadolimab may elicit a robust and long-lasting neutralizing polyclonal antibody response in COVID-19 patients, which may provide cross-protection against emerging variants of SARS-CoV-2.
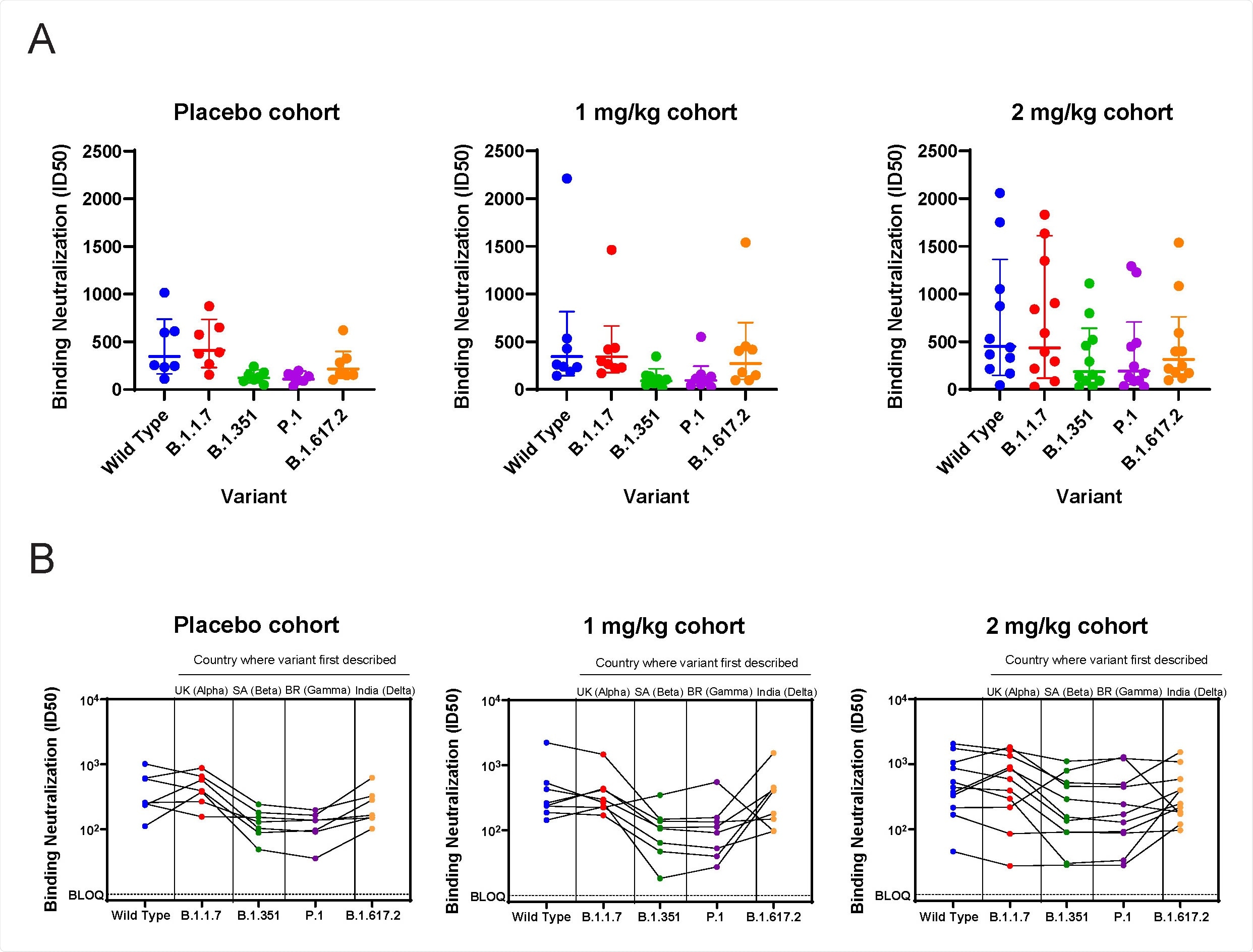
ACE2 / RBD blocking test. A) ID50 for serum samples tested against wild type (Wuhan) and variants using the ACE2-RBD blocking assay. B) As in A, with data showing each patient’s responsiveness to variants.
Randomized, double-blind, placebo-controlled trial
A multicenter, stratified study of mupadolimab plus standard of care (SOC) versus placebo plus SOC, in hospitalized patients with mild to moderate symptoms with COVID-19 was initiated. Here, all three treatment arms received intravenous mupadolimab – 2 mg / kg, 1 mg / kg, or a placebo given as a 5-10 minute infusion.
There were no drug-related side effects in patients receiving mupadolimab. All endpoints tended towards a more favorable outcome for mupadolimab; 93.3%, 85.7% and 81.1% of patients were alive and without respiratory failure in the 2 mg / kg, 1 mg / kg mupadolimab and placebo cohorts, respectively. Mupadolimab also performed better in terms of time to clinical improvement, time to lasting improvement, and time to discharge.
In addition, patients who received 2 mg / kg mupadolimab also had a higher cross-reactivity with variants B.1.351 and P.1 compared to those who received 1 mg / kg mupadolimab and placebo.
Mupadolimab causes prolonged retention of activated B cells in lymphoid organs and the thymus. In addition, this drug induces the secretion of cytokines involved in the differentiation of B cells, including CCL22. It is proposed that mupadolimab restores appropriate immune function in germinal centers (of impaired humoral immunity) of patients infected with SARS-CoV-2, either through its action on B cells or other CD73 positive cells.
The results described an exceptionally magnanimous and long-lasting antibody response with mupadolimab treatment, which offers a new approach to treat COVID-19 through stimulation of B cells.
*Important Notice
medRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link