[ad_1]
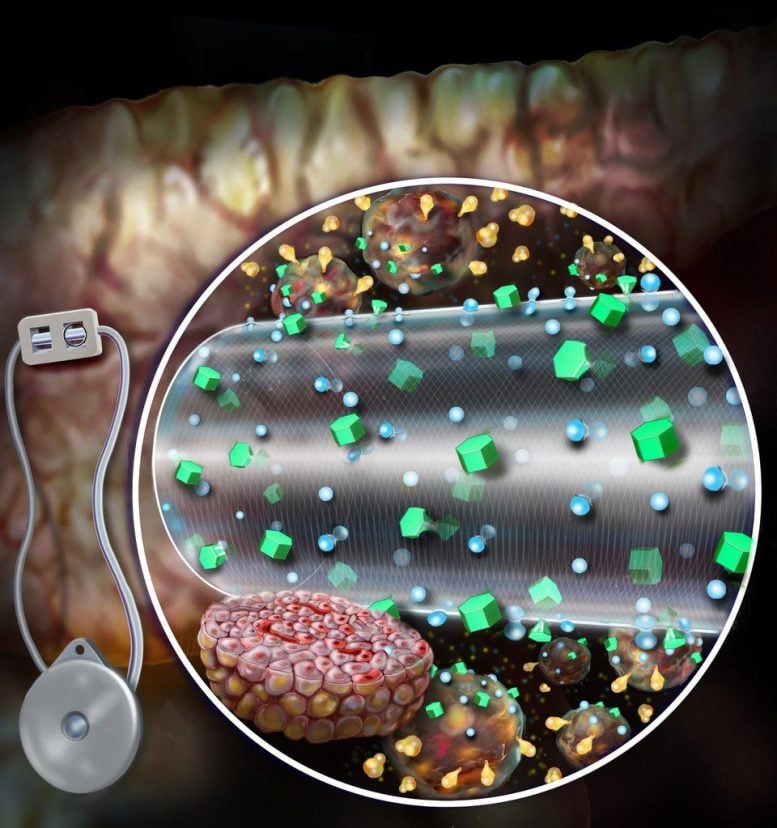
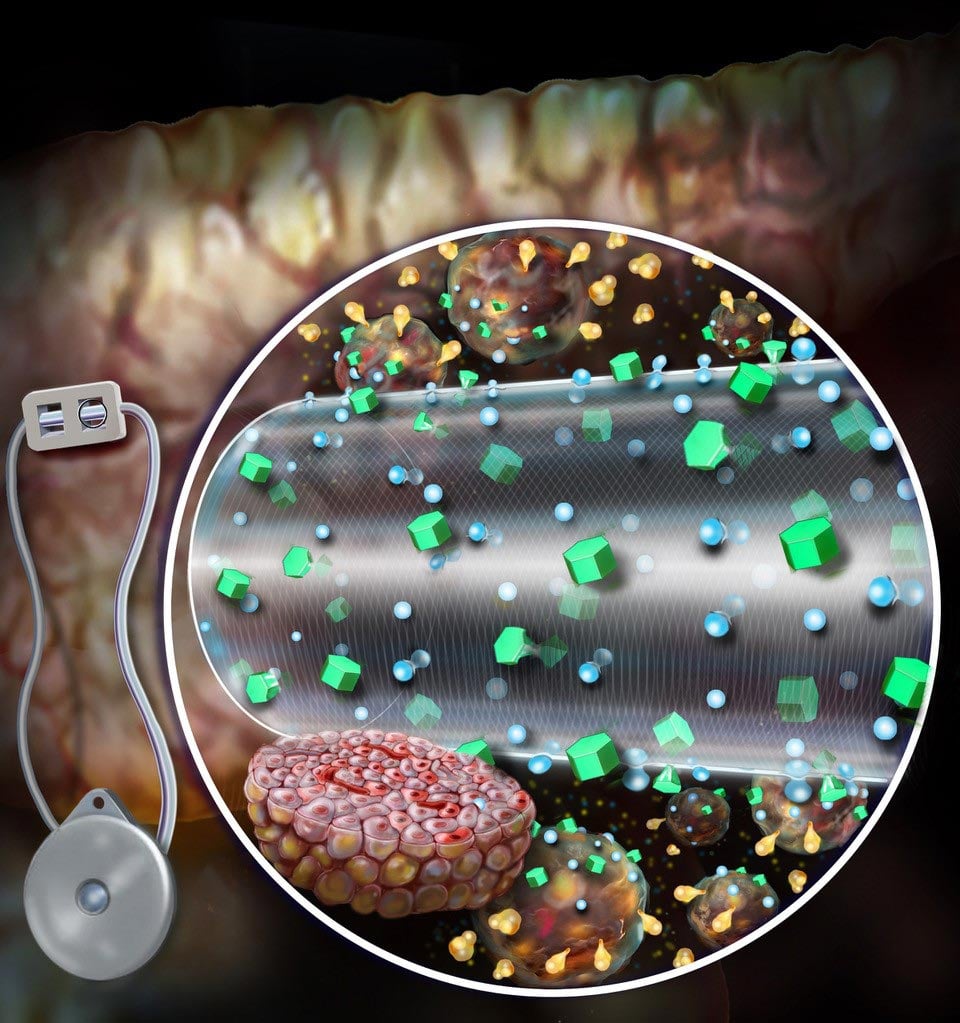
A convection-enhanced encapsulation device, as shown, improves nutrient transport, which improves survival and enhances the secretion of glucose-sensitive insulin from transplanted beta cells. Credit: Randal McKenzie
Brigham bioengineers have developed a convection-enhanced macroencapsulation device that offers the potential for faster and more effective treatment for people with type 1 diabetes.
More than 40 million people worldwide have type 1 diabetes (T1D), an autoimmune disease in which the insulin-producing β cells in the pancreas are destroyed by the immune system. Today, there are several new and emerging treatment methods for type 1 diabetes, including macroencapsulation devices (MED) – compartments designed to house and protect insulin-secreting cells. Like an armor around a knight, MED protects the cells inside from attack (from the host’s immune system) while allowing nutrients to come in and out so the cells can continue to survive. But MED have several limitations and scaling up such devices for use in humans has been a challenge. A team of researchers from Brigham and Women’s Hospital, in collaboration with colleagues at Harvard University and the University of Massachusetts Medical School, have designed a convection-enhanced MED (ceMED), which can continuously bathe the cells in the nutrients they need and improve cell carrying capacity, while increasing cell survival, glucose sensitivity and timely insulin secretion. In preclinical models, ceMED responded rapidly to blood sugar levels within two days of implantation. The results are published in Proceedings of the National Academy of Sciences.
“Thanks to recent advancements, we are getting closer and closer to an unlimited source of type cells that can respond to glucose by secreting insulin, but the next challenge is to get these cells into the body of a minimally invasively and will have longevity with maximum function, ”said corresponding author Jeff Karp, PhD, Principal Investigator and Distinguished Chair in Clinical Anesthesiology, Perioperative and Pain Medicine. “Our device demonstrated improved cell viability and minimal delay after transplantation. This is a strong preclinical proof of concept for this system.
Current MEDs depend on diffusion – nutrients diffuse through the outer membrane of the device and only a certain number of cells can receive nutrients and oxygen and, in turn, secrete insulin. CeMED was designed to deliver convective nutrients via a continuous flow of fluid to the encapsulated cells, allowing multiple layers of cells to grow and survive. The team’s prototype includes two chambers: an equilibrium chamber (EqC) which collects nutrients from the environment and a cell chamber (CC) which houses the protected cells. The EqC is enclosed in polytetrafluoroethylene – a semi-permeable membrane with pores that allow fluids to enter. An additional inner membrane surrounding the CC selectively allows nutrient transport and protects against immune responses. The infused fluids flow through a porous hollow fiber reaching the CC at a nutrient concentration similar to that of the tissue surrounding the implant. The hollow fiber allows insulin and glucose to pass freely but does not allow key immune molecules to attack the encapsulated cells.
“The application of islets derived from stem cells to treat autoimmune or type 1 diabetes has now evolved to find a method to protect cells from immune rejection and maximize their survival and function after transplantation,” said said co-author Doug Melton, PhD, of the Department of Stem Cells and Regenerative Biology at the Harvard Stem Cell Institute. “Convection-enhanced macroencapsulation may well be a viable approach to achieve all of these goals.”
The device offers many advantages over conventional insulin pumps and allows cells to secrete insulin on demand and quickly stop secreting insulin when blood sugar drops. In rodent models of type 1 diabetes, ceMED improved cell survival and insulin secretions and began to lower blood sugar as early as two days after transplantation.
“The ceMED device has the potential to be a stand-alone system that would not require constant refilling and replacement of insulin cartridges,” said lead author Kisuk Yang, PhD, former postdoctoral fellow at Karp Lab and now professor in the Bioengineering Division of Incheon National University in South Korea.
“Because of its responsiveness, this device and new approach to improving throughput may be particularly useful for ‘frail’ diabetics, people whose diabetes causes unpredictable fluctuations in blood sugar,” added Eoin O’Cearbhaill, PhD (now at University College Dublin, Ireland), a co-author who helped develop this concept while working as a postdoctoral fellow at Karp Lab. The team notes future directions that will need to be pursued to bring the device to the clinic, including increasing cell loading capacity and optimizing the perfused flow system for human use.
“Overall, these results highlight the significant advantages of ceMED over existing diffusion-based devices, including improved cell survival, reduced fibrous encapsulation which can compromise functionality over time and levels of. Faster on and off for insulin secretion, ”said Karp. “This approach has the potential to improve the success of β cell replacement therapies in helping many T1D patients and their families manage this difficult disease. “
Reference: “A macroencapsulation device enhanced by therapeutic convection to improve cell viability and insulin secretion” by Yang K et al., September 6, 2021, Proceedings of the National Academy of Sciences.
DOI: 10.1073 / pnas.2101258118
Funding: This work was supported by the Juvenile Diabetes Research Foundation (3-SRA-2013-282), and the National Institutes of Health (grant R01 HL095722 and U01DK104218), and the research grant from National University of Incheon in 2021.
[ad_2]
Source link