
[ad_1]
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has currently infected more than 205 million people, with the current total number of deaths from coronavirus disease 19 (COVID-19) rising to more than 4 , 33 million. Medicines for SARS-CoV-2 are urgently needed to save lives and revive economies around the world. However, the effectiveness of drugs currently in development is limited by several factors, including low specificity. Although therapeutic antibodies have been identified as having higher specificity against SARS-CoV-2, they are difficult to mass produce due to high production costs and low yields.
In a research article recently published in the journal eLife, researchers from several American institutions have developed a drug candidate based on nanobodies that effectively prevents and suppresses severe infection with SARS-CoV-2 in animals.

If validated in human trials, the drug may present itself as a more suitable candidate for COVID-19 treatment than other therapeutic antibodies due to its thermostability and ability to be produced easily. Moreover, by using the drug nanobody, the infection could be prevented and treated in the short term.
Nanosota-1 drug
Globally, scientists are studying the suitability of nanobodies as treatment candidates for COVID-19. Nanobodies are single domain antibodies derived from heavy chain antibodies only found in members of the camelid family, such as alpacas and llamas. Although smaller than conventional antibodies, they bind with high affinity and specificity.
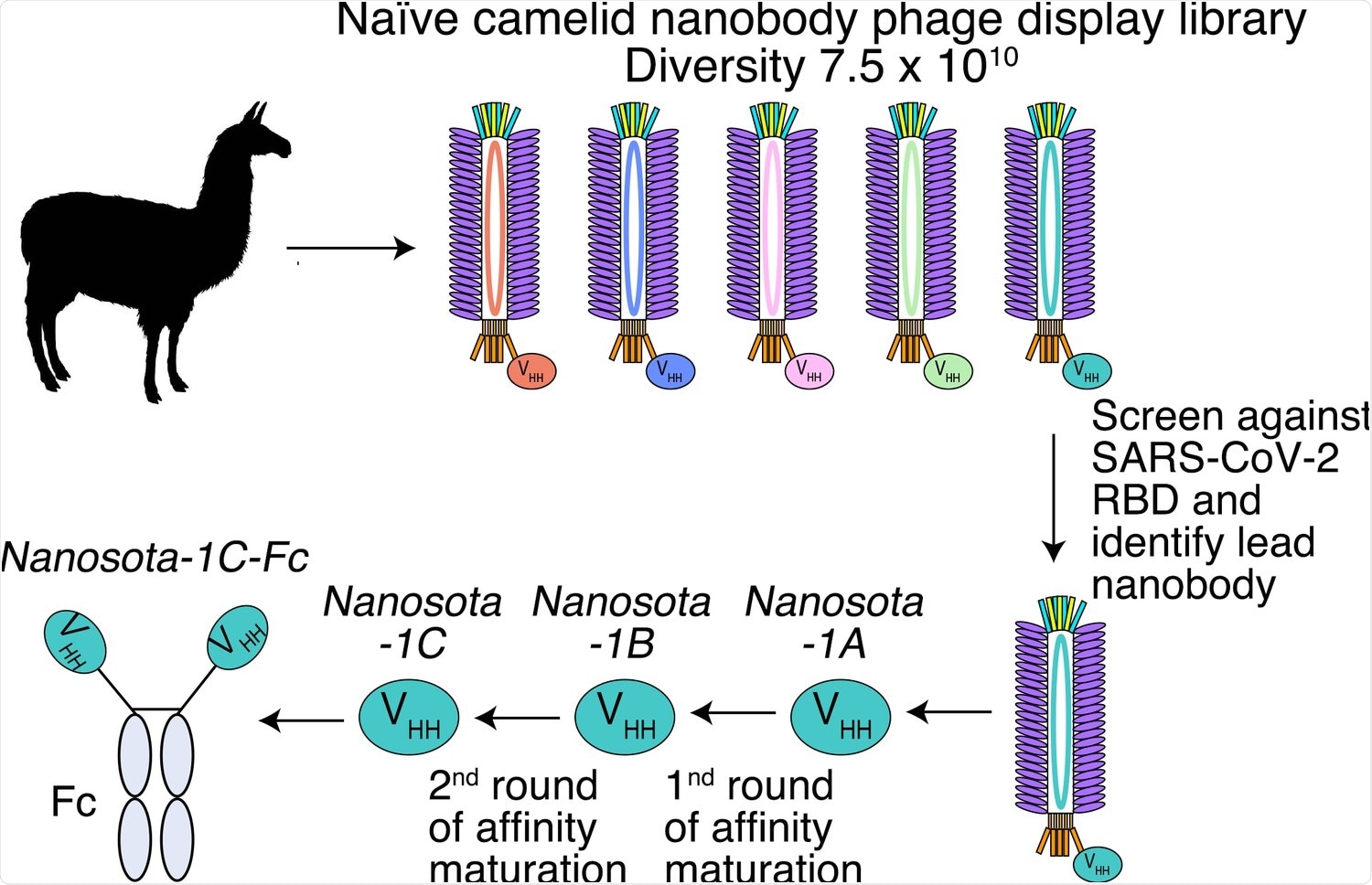
Construction of a phage display library of camelid nanobodies and use of this library for screening anti-SARS-CoV-2 nanobodies.
Nanosota antibodies have been shown experimentally to bind to the receptor binding domain (RBD) of the SARS-CoV-2 spike protein with high affinity, blocking the protein’s binding to the human L-converting enzyme. angiotensin 2 (ACE2) and preventing entry into cells. This principle underlies the way the drug Nanosota-1 works because the drug contains nanobodies that bind to the SARS-CoV-2 spike protein to block infection.
The study
In the present study, researchers developed a series of anti-SARS-CoV-2 nanobody drug candidates by screening a phage display library of camelid species nanobodies against the viral receptor binding domain (RBD).
One of these candidates was the nanosota-1 series of antibodies, which were shown to competitively bind SARS-CoV-2 RBD with ACE2 and bind more strongly. Thus, inhibiting SARS-CoV-2 infection by preventing entry of cells.
In particular, the best and best performing drug candidate, Nanosota-1C-Fc, has shown preventive and therapeutic efficacy against SARS-CoV-2 infection in hamster and mouse models. In addition, the lead candidate showed excellent in vitro stability through temperatures and very good bioavailability and biodistribution compared to other members of the series. Thus, the Nanosota-1 series is an ideal drug candidate for targeting RBD, as it suppresses and inhibits SARS-CoV-2 viral particles.
“It is important to note that we have shown that the efficacy of Nanosota-1C-Fc is not limited to in vitro experiments, but translates directly into in vivo experiments by demonstrating its efficacy in animal models,” explained researchers in the study.
Conclusion
The characteristics of the drug, including excellent in vitro thermostability, high tissue bioavailability and good in vivo stability, are crucial for the implementation of the drug. Nanosota-1C-Fc as a potential treatment for COVID-19. Different from traditional antibody drugs in development, Nanosota-1 can be produced at high yields without compromising its stability over wide temperature ranges. In addition, the nanobody drug candidate can be produced cost-effectively, while remaining stable during manufacture, transport and storage. Therefore, it could help make up for the shortage of current monoclonal antibody therapies and ultimately help treat COVID-19.
Source:
Journal reference:
Source link