
[ad_1]
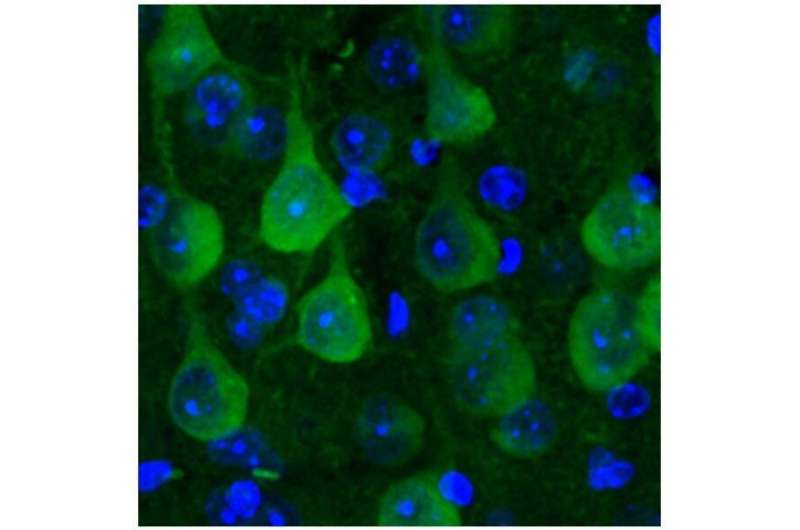
In healthy mouse neurons, the protostasis sensor (in green) is distributed evenly. Credit: MPI of Neurobiology / Blumenstock
Proteins are the “tools” of our cells, they are essential for all vital tasks. However, they can only do their job if they bend properly and adopt their respective very specific 3D structure. To ensure that nothing goes wrong with the folding process, it is strictly monitored in the cell. The consequences of faulty quality control can be seen, for example, in the deposition of misfolded proteins in neurodegenerative diseases such as Alzheimer’s disease. Researchers from the Max Planck Institutes of Neurobiology and Biochemistry have now developed a line of mice that for the first time makes visible the steady state of proteins in the brain of mammals. In this way, protein quality control processes can now be studied in more detail in healthy and diseased neurons.
Proteins perform all the important tasks of our body: they transport substances, protect against disease, support the cell and catalyze chemical reactions, to name a few. With the instructions for building our genetic code, each protein can be produced as a long chain of amino acids. However, this is not the end of the story: to perform their vital functions, proteins must fold into complex 3D structures.
Each cell contains a whole machinery that helps proteins to fold, corrects folding errors and eliminates misfolded proteins. As a quality control, the system thus contributes to proteostasis, the controlled function of all proteins.
In healthy cells, this quality control works very well. With age, however, it gradually deteriorates. It can become a problem, especially for nerve cells. These cells do not renew themselves and are therefore dependent on stable protein function throughout their life. Indeed, neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease or Huntington’s disease have in common that certain misfolded proteins overload the quality control system and are not eliminated. These proteins accumulate, clump together and eventually form deposits in brain tissue. Depending on the disease, this can lead to impaired memory or muscle control, with no chance of a cure so far. The ability to improve the quality control of neurons could thus present a promising therapeutic option.
New Mouse Line
In order to further study quality control flaws in individual diseases, scientists led by Irina Dudanova developed a new line of mice. With these animals, the state of proteostasis can be visualized in the brain of mammals for the first time.
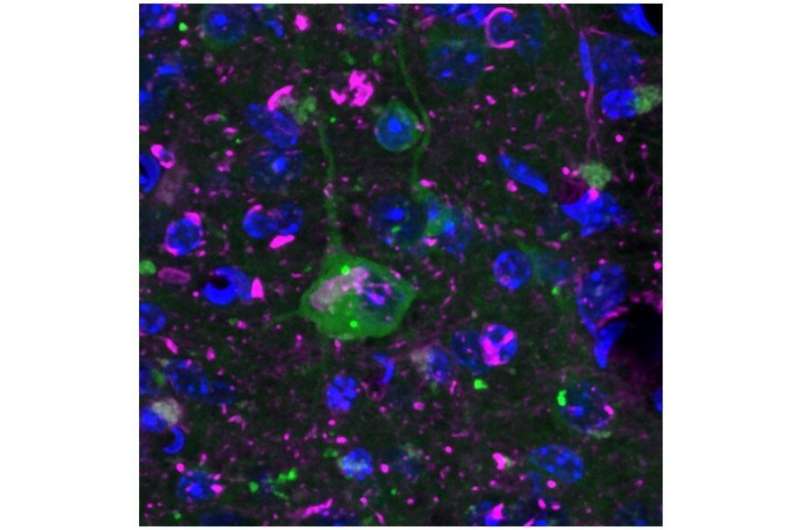
In cells that partially mimic Alzheimer’s disease, the sensor protein clumps together (middle cell, green dots), indicating an interruption in protein quality control. In addition, deposits of misfolded proteins, characteristic of Alzheimer’s disease, are visible in purple. The nuclei are colored blue. Credit: MPI of Neurobiology / Blumenstock
The researchers introduced the protein that normally makes fireflies glow into mouse neurons. Optimized at the body temperature of the beetle, the protein needs constant help to fold in “warmer” mammals. Only then can it adopt its correct structure and produce light. In order to accurately track the location of the luminescent protein in the cell, the scientists further marked it with a dye. In this way, they showed that the protein is evenly distributed and shines in healthy neurons. However, if the protein quality control is overloaded, the beetle protein will clump together and no longer glow as brightly. The beetle protein therefore serves as a protostasis sensor.
The researchers then crossed the newly developed mouse line with mice that represent different neurodegenerative diseases. In mice showing signs of Alzheimer’s disease, luminescent protein formed clumps, signaling strong disruption of proteostasis. Interestingly, this was not the case in Chorea Huntington mice. Irina Dudanova says: “The different results were quite surprising. When we took a closer look at the possible reasons, we found that the misfolded proteins themselves and their location in the cell play an important role.
Variation within the cell
While the misfolded protein in the Alzheimer’s model forms deposits in the cell body, it agglutinates in the cell nucleus of Huntington mice. As a result, protein quality control and its ability can vary widely within a cell. “This shows how complex protein quality control is and how different its alterations can be in individual neurodegenerative diseases,” says Irina Dudanova.
With the new mouse line, scientists now have a tool to specifically study this complexity, both in healthy and diseased neurons. Irina Dudanova and her team plan to study other neurodegenerative diseases and find out if different types of brain cells are affected at different rates. In addition, the mouse line could help assess the effectiveness of different therapies for neurodegenerative diseases.
Researchers identify key players in mysterious protein quality control process
Sonja Blumenstock et al, Fluc-EGFP reporter mice show differential alterations in neuronal proteostasis during aging and disease, The EMBO Journal (2021). DOI: 10.15252 / embj.2020107260
Provided by the company Max Planck
Quote: Defective Quality Control in Brain Traced by Misfolded Proteins (2021, Aug 20) retrieved Aug 20, 2021 from https://phys.org/news/2021-08-flawed-quality-brain-misfolded-proteins .html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link