
[ad_1]
A debate is raging around proposals for the introduction of a second Ebola vaccine in the Democratic Republic of Congo, currently plagued by the most serious epidemic.
DR Congo's Minister of Health, Dr. Oly Ilunga, who resigned after being deprived of management of the country's response to Ebola, said the current vaccine was the only one to have proven effective and that an opposition MP had said that the new vaccine had not been tested yet. and fears that the inhabitants of the country will be used as guinea pigs.
Health experts believe that the second vaccine is safe and could be an important tool in curbing the spread of the virus.
So what are the concerns and are they justified?
Tried and tested?
It has been tested on 6,000 people and "has demonstrated exceptional safety," says Professor Peter Piot, a leading specialist in Ebola Virus Disease and Director of the London School of Hygiene & Tropical Medicine, badociated with the development of the pharmaceutical company Johnson & Johnson. of the vaccine.
Studies have shown that, although the drug is still in the experimental phase and has not yet been tested in Ebola patients, it has been shown to be very effective in primate testing (genetically related animals of the human).
The only way to test it on humans is to use it in an epidemic scenario because it would not be prudent to test the drug on volunteers infected with the virus during one test clinical.
This is how the first vaccine – from the pharmaceutical company Merck & Co – was successfully deployed in Guinea in 2015.
It has been deployed for a "compbadionate use" that allows the use of an unlicensed drug (obtaining a license can take years, or even decades) when it is not used. there is no other option, but only with the permission of the government of the affected country.
Data from the World Health Organization (WHO) show that the Merck vaccine has an effectiveness rate of 97.5% for immunized people, compared to those who are not.
The WHO says the vaccine is safe and effective against the Ebola virus, but additional testing is still needed to obtain a license.
So we are in the same place with the new vaccine as in 2015 with the current vaccine: there is substantial evidence of its safety and efficacy, but it has not been tested during an outbreak, and it has not been tested. has not been tested. t been allowed.
 Copyright of the imageGETTY IMAGES
Copyright of the imageGETTY IMAGES
Image caption A woman subjected to Ebola screening
Is there enough vaccine?
If there is already a vaccine whose effectiveness has been proven against Ebola, why not deploy more?
In July, the WHO Emergency Committee declared that it "recognized the lack of supply" of the Merck vaccine.
Dr. Josie Golding of the Wellcome Trust, says that it is likely that there will not be enough vaccine to cope with the current epidemic.
"If this is the case, it would have devastating consequences.We firmly believe that it is urgent to deploy and evaluate a second Ebola vaccine, developed by Johnson and Johnson."
In the short term, the vaccine doses available may be sufficient, but not if the epidemic persists.
The pharmaceutical firm Merck says that there is enough vaccine to immunize nearly 500,000 people at the current dose and is producing more.
About 1.5 million doses of vaccine are available.
There are approximately 10 million people in the two affected provinces combined.
The current vaccine is only administered to health workers and people who may have been exposed to the virus.
So, if the DR Congo government wants to deploy vaccination in a larger area, it will need more supplies.
Those who advocated the use of the new Johnson & Johnson vaccine had proposed using it to create a protective wall and vaccinate people living outside the epidemic zone.
It is hoped that this will prevent the virus from spreading.
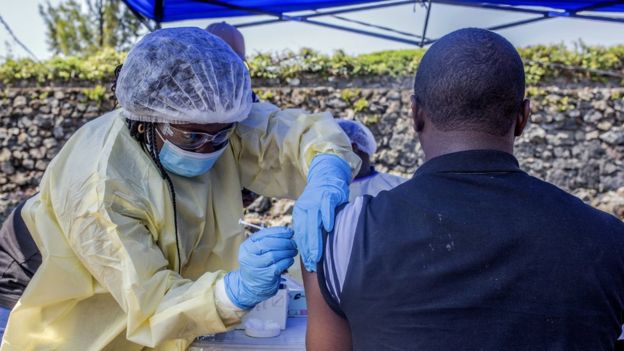 Image Copyright GETTY IMAGES
Image Copyright GETTY IMAGES
Image caption A man receives a vaccine in DR Congo
Mistrust of the community
The government of DR Congo has expressed concerns about the complexity of using two vaccines together in the response.
He says there is a risk of increasing confusion and mistrust among the affected communities.
The fight against the current Ebola outbreak has been marked by the mistrust of the community towards the response.
There is also concern that the new vaccine – which requires two injections 56 days apart – may be difficult to administer in a region where the population is highly mobile and insecure.
[ad_2]
Source link