.jpg)
[ad_1]
Portuguese researchers have provided evidence that could explain why infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causes mild or even asymptomatic illness in some people, but severe coronavirus disease 2019 (COVID- 19) in others.
Salome Pinho from the University of Porto and colleagues have shown that circulating T cells exhibit a specific ‘glycan switch’ following infection with SARS-CoV-2 and that this change is more pronounced in asymptomatic individuals compared to symptomatic individuals.
The researchers say that this change in the glycosylation profile of T lymphocytes appears to be triggered by a serum inflammatory factor, the identification of which could lead to a potential new biomarker and a therapeutic target.
The team also demonstrated that circulating monocytes in asymptomatic patients exhibit upregulated expression of a protein called DC-SIGN (Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin).
In addition, a higher level of DC-SIGN expression in monocytes correlated with a better patient prognosis.
“These new findings pave the way for the identification of a novel glycan-based response in T cells that may confer protection against SARS-CoV-2 infection in asymptomatic patients, highlighting a new prognostic biomarker and a potential therapeutic target, ”the team writes.
A pre-printed version of the research paper is available on the medRxiv* server, while the article is subject to peer review.
.jpg)
People are affected by SARS-CoV-2 infection widely
The course of illness after infection with SARS-CoV-2 is highly variable, ranging from mild or asymptomatic illness to severe illness characterized by pneumonia, acute respiratory distress syndrome and multi-organ failure.
Although severe illness usually develops in older people, some relatively young and healthy people also develop severe COVID-19.
Pinho and colleagues say a better understanding of patient-specific immune responses is urgently needed to help identify prognostic biomarkers and treatment targets, and to improve patient stratification for vaccination.
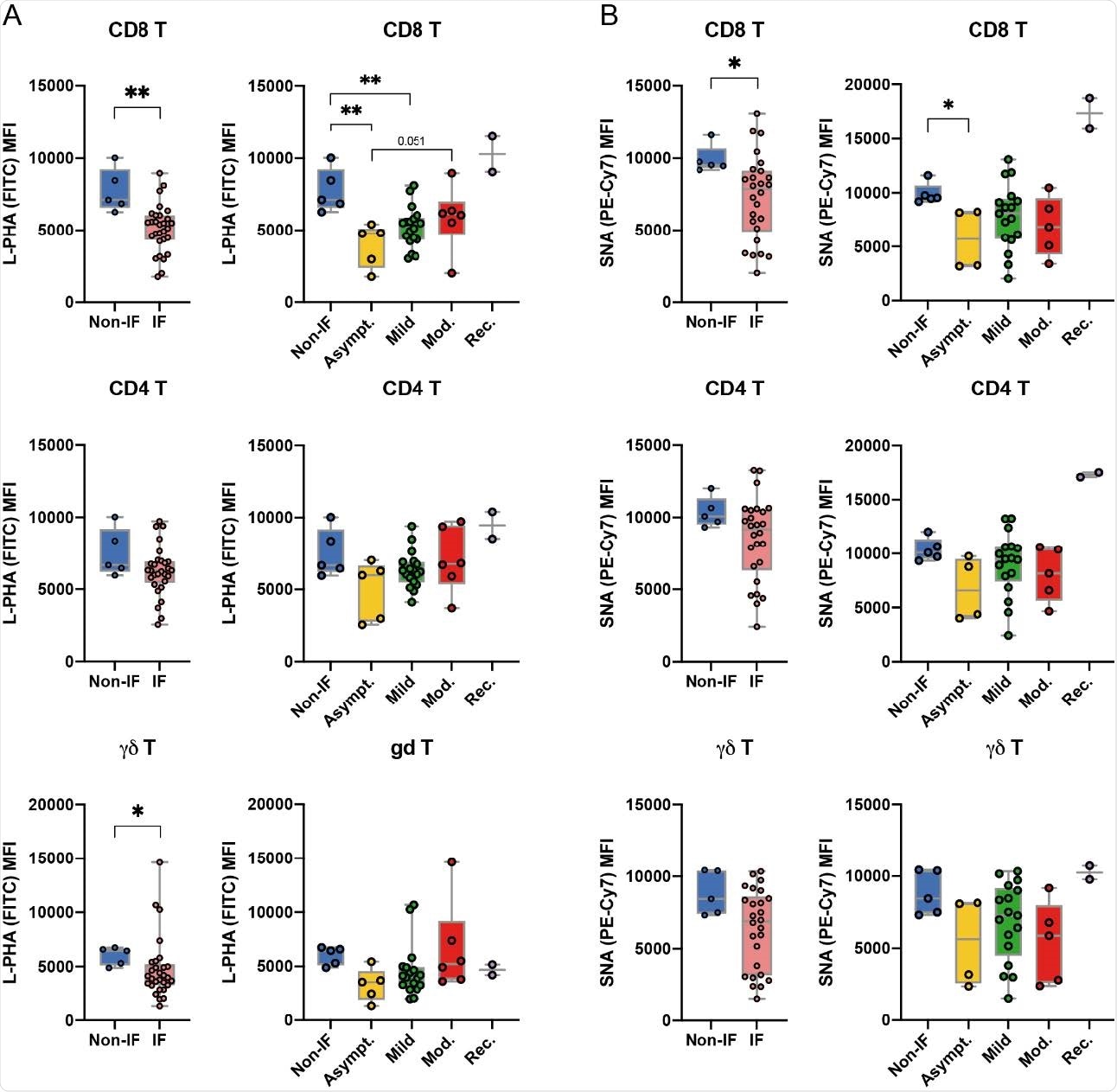
The glycoprofile of peripheral T cells is altered upon infection with SARS-CoV-2 at the time of diagnosis. Levels (mean intensity fluorescence, MFI) of (A) L-PHA lectin binding, detection of branched N-glycans β1,6-GlcNAc and binding of (B) SNA lectin, detection of a-2 sialylation , 6, in CD8 +, CD4 + and γδ T cell subsets from uninfected donors (non-IF; n = 5) and from infected patients (IF; n = 30). (A) L-PHA and (B) SNA lectin binding levels for each disease severity group: asymptomatic (asymptomatic; n = 5), mild (n = 18), moderate (Mod .; n = 6 ) and restored (Rec; n = 2) Each point represents a patient. The Mann-Whitney t test was performed to assess statistically significant differences between each pair of groups. * p-value <0.05, ** <0.005.
Where do glycans come from?
Glycosylation refers to the controlled enzymatic addition of glycans (carbohydrates) to proteins or lipids present on cells. Glycans are important regulators of immune cell function and determine T cell activation and differentiation.
Pinho and the team have previously shown that glycans modulate T cell activation thresholds associated with immunopathogenesis in autoimmune diseases and cancer.
As is the case with most viruses, the viral envelope of SARS-CoV-2 is highly glycosylated with structures such as oligomannose and branched N-glycans.
These structures are specifically recognized by glycan-binding proteins such as DC-SIGN, which promote viral recognition and elimination.
However, “the glycosylation profiles of immune cells in individuals infected with SARS-CoV-2 and their impact on their effector functions remain completely unknown,” the team explains.
What was the current study?
Pinho and his colleagues analyzed blood samples taken from 32 infected patients hospitalized between May 2020 and July 2020 and compared them with samples taken from healthy and uninfected controls.
Researchers have shown that infection with SARS-CoV-2 induces a change in the glycosylation profile of circulating T cells.
More specifically, T cells from infected individuals exhibited a decrease in branched structures of N-glycan (in particular on CD8 + and γδ T cells), compared to T cells from uninfected individuals.
This specific “glycan change” was more pronounced in T cells of asymptomatic patients than in those of symptomatic patients.
The results suggest that this glycan change was triggered by the onset of a serum inflammatory factor shortly after infection.
“It is essential to identify the critical serological factor (s) that drive this glycoprogramming of T cells, as a potential new biomarker and therapeutic target,” said Pinho and colleagues.
The study also demonstrated that patients with a good prognosis exhibited upregulated expression of DC-SIGN in circulating monocytes.
“This study reveals the identification of a specific glycosignature of T cells, as well as a prognostic biomarker in COVID-19,” the team writes.
What are the implications of the study?
“Here we propose that the immune response against SARS-CoV-2 infection appears to be influenced by the glycosylation profile of circulating T cells, which defines their efficient functions,” the researchers write.
Pinho and his colleagues say the results pave the way for identifying a specific blood glycosignature that could be used to stratify patients at diagnosis based on their risk of disease worsening.
“This will certainly help improve the vaccination strategy and patient risk stratification, optimize the efficient allocation and management of health resources such as ventilators and intensive care facilities,” the team concludes.
*Important Notice
medRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behaviors or be treated as established information.
Source link