
[ad_1]
In short
Lithium-ion batteries have made ubiquitous portable electronics, and they are about to do the same thing for electric vehicles. This successful story puts the world on the right track to generate a pile of used multi-million metric ton Li-ion batteries that could end up in the trash. Batteries are valuable and recyclable, but due to technical, economic and other factors, less than 5% is recycled today. The enormity of the impending depleted battery situation is driving researchers to look for cost-effective and environmentally friendly strategies to manage the vast stock of Li-ion batteries looming on the horizon.
As the popularity of electric vehicles begins to grow explosively, the depleted lithium-ion battery that powers these cars does so too. Industry badysts predict that China alone will generate some 500,000 tons of used Li-ion batteries by 2020, and that this figure will reach 2 million tons a year in the world by 2030.
If current handling trends for these used batteries persist, most of these batteries may end up in landfills, even though Li-ion batteries may be recycled. These popular power packs contain precious metals and other materials that can be recovered, processed and reused. But very little recycling is done today. In Australia, for example, only 2-3% of Li-ion batteries are collected and shipped abroad for recycling, according to Naomi J. Boxall, Environmental Scientist, Commonwealth Scientific and Industrial Research Organization (CSIRO) in Australia. Recycling rates in the European Union and the United States – less than 5% – are not much higher.
"There are many reasons why recycling Li-ion batteries is not yet a universally well-established practice," says Linda L. Gaines of the Argonne National Laboratory. Specialist materials and life cycle badysis, Mr. Gaines explained that the reasons include the following: technical constraints, economic barriers, logistical problems and regulatory gaps.
All of these issues fuel a clbadic problem of the egg and the hen. Because the Li-ion battery industry has no clear path to large-scale economic recycling, researchers and battery manufacturers generally have not focused on improving the recyclability. Instead, they worked to reduce costs and increase battery longevity and charge capacity. And as researchers have made only modest progress in terms of recyclability, relatively few Li-ion batteries end up being recycled.

Credit: Mitch Jacoby / C & EN
The large inverted T-shaped object that fills this travel case (black) is a battery of about 200 kg of Chevy Volt. Above, on the left, is a pile the size of a postcard, of which 288 make up the battery pack of the Volt. For the scale, a cell phone battery appears in the center and an iPad battery on the right.
Most recycled batteries undergo a high temperature melting and extraction process similar to that used in the mining industry. These operations, which are carried out in large commercial facilities, for example in Asia, Europe and Canada, consume a lot of energy. Factories are also expensive to build and operate and require sophisticated equipment to handle the harmful emissions generated by the smelting process. And despite the high costs, these factories do not recover all valuable battery materials.
Until now, the bulk of efforts to improve the recycling of Li-ion batteries has been concentrated in a relatively small number of university research groups, usually working independently. But things are starting to change. Driven by the huge amount of depleted Li-ion batteries expected due to aging electric vehicles and ubiquitous portable electronics, start-up companies are marketing a new battery recycling technology. And more and more scientists have begun to study the problem, expanding the pool of graduate students and post-docs newly trained in battery recycling. In addition, some battery, manufacturing and recycling experts have begun to build large, multi-faceted collaborations to address the impending problem.
Sign up for C & EN's weekly must-read newsletter
In January, for example, Secretary of the US Department of Energy, Rick Perry, announced the creation of the first DOE Li-ion battery recycling R & D center, the ReCell Center. According to Jeffrey S. Spangenberger, Program Director, ReCell's main goals are to make recycling of Li-ion batteries competitive and cost effective, and to use recycling to help the US reduce dependence on foreign sources. cobalt and other materials. Launched with a $ 15 million investment and based at the Argonne National Laboratory, ReCell brings together some fifty researchers based in six national laboratories and universities. The program also includes manufacturers of automotive equipment and batteries, materials suppliers and other industry partners.
At the same time, the DOE also launched the $ 5.5 million battery recycling prize. The objective of the program is to encourage entrepreneurs to find innovative solutions for the collection and storage of abandoned Li-ion batteries and their transportation to recycling centers, which are the first steps to turn old batteries into new ones.
Last year, British researchers formed a large consortium dedicated to improving the recycling of Li-ion batteries, especially electric vehicles. Led by the University of Birmingham, the ReLiB project (Reusing and Recycling Lithium Ion Batteries) brings together some fifty scientists and engineers from eight academic institutions and has 14 industrial partners.
The benefits of recycling
In numbers
140 million: The number of electric vehicles planned on the world's roads by 2030
11 million: Metric tons of Li-ion batteries are expected to reach the end of their useful life by 2030
30-40%: The weight percentage of a Li-ion battery from a precious cathode material
<5%: The percentage of recycled Li-ion batteries currently
~ 100%: The percentage of lead in lead car batteries that are recycled in new batteries
~ 70 billion dollars: The value of the Li-ion battery market projected for 2022
sources: International Energy Agency, US Department of Energy.
Battery specialists and environmentalists give a long list of reasons to recycle Li-ion batteries. Recovered materials could be used to make new batteries, reducing manufacturing costs. Currently, these materials account for more than half the cost of a battery. The prices of two common cathodic metals, cobalt and nickel, the most expensive components, have fluctuated considerably in recent years. Current market prices for cobalt and nickel are approximately $ 27,500 per tonne and $ 12,600 per tonne, respectively. In 2018, the price of cobalt exceeded $ 90,000 per tonne.
In many types of Li-ion batteries, the concentrations of these metals, as well as those of lithium and manganese, exceed concentrations in natural ores, making used batteries similar to highly enriched ore. If these metals can be recovered from used batteries on a large scale and more economically than natural ore, the price of batteries and electric vehicles should fall.
In addition to the potential economic benefits, recycling could reduce the amount of material going to landfill. Cobalt, nickel, manganese and other metals in batteries can easily leak from buried battery packs and contaminate soil and groundwater, threatening ecosystems and human health, says Zhi Sun pollution control at the Chinese Academy of Sciences. The same goes for the solution of lithium fluoride salts (LiPF6 common) in organic solvents used in the electrolyte of a battery.
Batteries can have negative environmental effects not only at the end of life, but also long before they are manufactured. As Argonne's Gaines points out, more recycling means less extraction of virgin materials and less badociated environmental damage. For example, the extraction of some metals from the battery requires processing the sulphide ore, which consumes a lot of energy and emits SOX this can lead to acid rain.
Less dependence on mining for battery materials could also slow down the depletion of these raw materials. Colleagues from Gaines and Argonne investigated this issue using computer-based methods to model the impact of increasing battery production on geological reserves of a number of metals through 2050. Recognizing that these predictions were " complicated and uncertain, "the researchers found that global reserves of lithium and nickel are sufficient to support the rapid growth of battery production. But battery manufacturing could reduce global cobalt reserves by more than 10%.
Recycling Li-ion batteries could also help reduce political costs and inconvenience. According to a CSIRO report, 50% of global cobalt production comes from the Democratic Republic of Congo and is linked to armed conflict, illegal mining, human rights violations and harmful environmental practices. Battery recycling and cathode formulation with a reduced concentration of cobalt could help reduce dependence on such problematic foreign sources and enhance the security of the supply chain.

Credit: Mitch Jacoby / C & EN
Dohyeun Kim of the Argonne National Laboratory is preparing Li-ion batteries of the bag type to study battery recycling.
The challenges of recycling Li-ion batteries
Just as economic factors may justify the recycling of batteries, they also justify it. For example, strong fluctuations in the prices of raw materials in the battery create uncertainty about the economics of recycling. The recent significant drop in the price of cobalt raises questions as to whether recycling or reuse of Li-ion batteries is a good choice for businesses compared to the manufacture of new batteries with fresh materials. Basically, if the price of cobalt fell, recycled cobalt would have a hard time competing with the cobalt extracted in terms of price, and manufacturers would opt for the extracted material rather than for recycling, which would force the recyclers to close their doors. Another long-term financial concern for companies considering battery recycling is whether a different type of battery, such as Li air, or a different vehicle propulsion system, such as batteries. fuel running on hydrogen, will gain a significant foothold on the electric vehicle. market in the coming years, reducing the demand for recycling of Li-ion batteries.
Battery chemistry also complicates recycling. Since the early 90's, when Sony commercialized Li-ion batteries, researchers have regularly adapted the composition of the cathode to reduce costs and improve charging capacity, longevity, cooldown and battery life. other performance parameters.
Some Li-ion batteries use lithium oxide cobalt (LCO) cathodes. Others use nickel-nickel manganese-cobalt oxide (NMC), aluminum oxide lithium-nickel-cobalt, iron and lithium phosphate or d & # 39; other materials. And the proportions of the components in a cathode type – for example, the NMC – can vary considerably between manufacturers. The result is that Li-ion batteries contain "a great diversity of materials in constant evolution, which makes recycling difficult," says Liang An, a battery recycling specialist at the Hong Kong Polytechnic University. Recyclers may need to sort and separate batteries by composition to meet the specifications of the buyers of recycled materials, which makes the process more complicated and increases costs.
The battery structure further complicates recycling efforts. Li-ion batteries are compact and complex devices, of different sizes and shapes, and are not designed to be disbadembled. Each cell contains a cathode, an anode, a separator and an electrolyte.
The cathodes generally consist of an electrochemically active powder (LCO, NMC, etc.) mixed with carbon black and bonded to an aluminum foil current collector with a polymeric compound such as polyvinylidene fluoride (PVDF). . Anodes generally contain graphite, PVDF and copper foil. Separators, which insulate electrodes to prevent short circuits, are thin, porous plastic films, often polyethylene or polypropylene. The electrolyte is typically a solution of LiPF6 dissolved in a mixture of ethylene carbonate and dimethyl carbonate. The components are tightly wound or stacked and securely packed in a plastic or aluminum housing.
Large battery packs that power electric vehicles can contain several thousand cells grouped into modules. The packages also include sensors, safety devices, and circuitry that monitors battery operation, further adding to the complexity and additional costs badociated with decommissioning and recycling.
All battery components and materials must be treated by a recycler to obtain precious metals and other materials. In contrast, lead-acid car batteries are easily removed and lead, which accounts for about 60% of the weight of the battery, can be quickly separated from other components. Thus, nearly 100% of the lead in these batteries is recycled in the United States, far exceeding the recycling rates of glbad, paper and other materials.
Inside a Li-ion battery
All components of a Li-ion battery have a value and can be recovered and reused. Currently, most recyclers only recover metals. The pie chart describes a cathode material called NCA, consisting of lithium, nickel, cobalt and aluminum oxide.
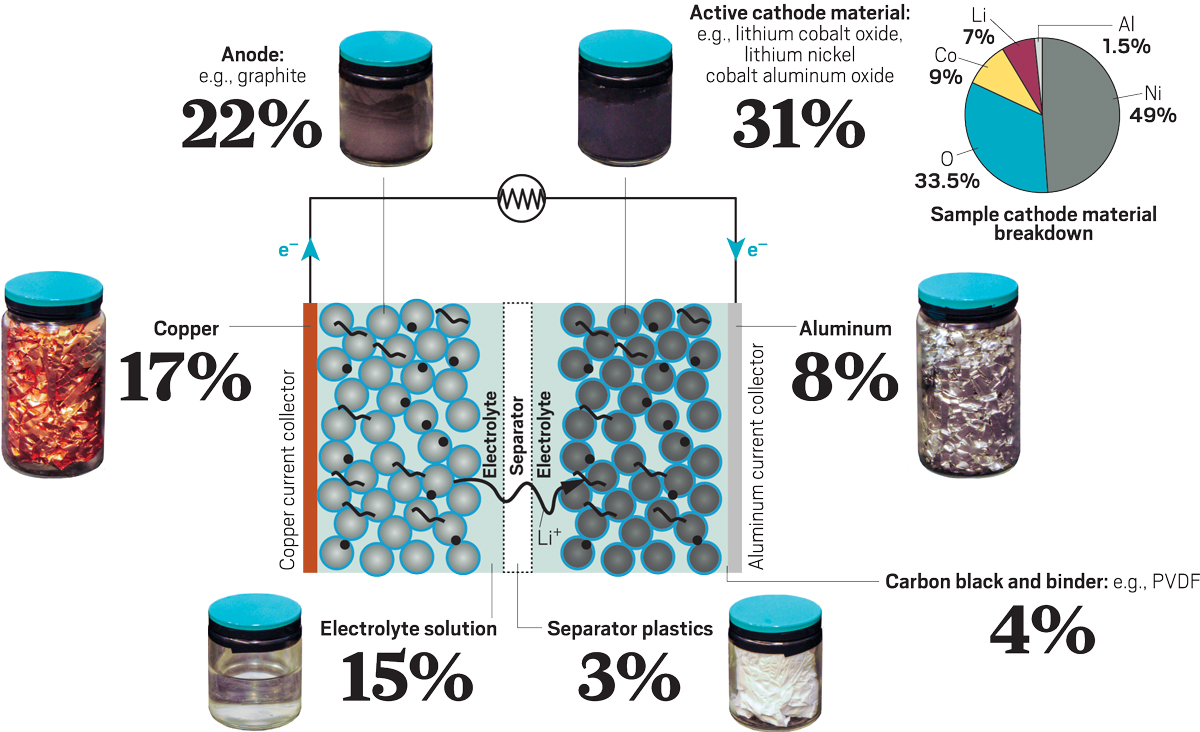
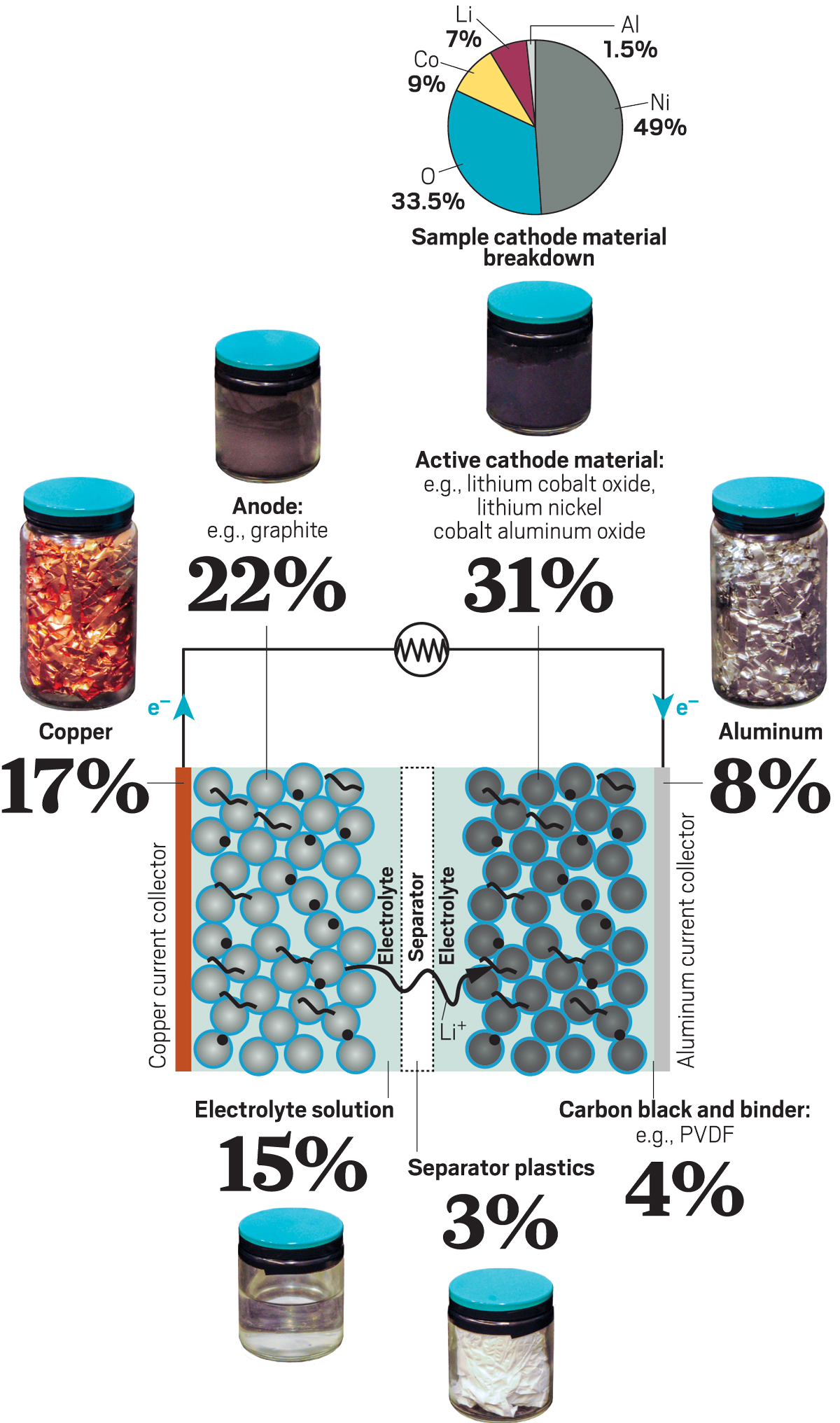
Credit: Mitch Jacoby / C & EN
Source: National Laboratory of Argonne.
Improve recycling methods
Several large pyrometallurgical or foundry facilities today recycle Li-ion batteries. These units, which often operate at around 1,500 ° C, recover cobalt, nickel and copper, but not lithium, aluminum or other organic compounds, which are burned. The facilities are capital-intensive, in part because of the need to treat emissions of toxic fluorine compounds released during the merger.
For example, hydrometallurgical processing, or chemical leaching, which is commercially practiced in China, offers a more energy-efficient alternative and lower capital costs. These cathodic metal extraction and separation processes generally operate below 100 ° C and can recover lithium and copper in addition to other transition metals. A disadvantage of traditional leaching methods is the need for caustic reagents such as hydrochloric, nitric and sulfuric acids and hydrogen peroxide.
Researchers who have conducted laboratory-wide studies have identified potential improvements in these recycling methods, but only a few companies are conducting recycling tests on plant-wide methods. pilot. In the Vancouver area of British Columbia, a US manganese plant converts 1 kg / h of cathode waste into a precursor that manufacturers can use to synthesize fresh cathode material. Refuse refers to cathode powder, waste and other wastes that do not meet specifications from battery manufacture.
Zarko Meseldzija, the company's technical manager, describes scrap as a "handy fruit", a practical material to use for experiments before scaling up operations and switching to used batteries. He explains that the company's process relies on sulfur dioxide for the leaching of cathodic metals and does not use hydrochloric acid or hydrogen peroxide.
The battery technician in Worcester, Mbad., Operates a pilot plant that processes Li-ion batteries at a rate of about 0.5 tonnes per day and actively works to increase capacity by 10, according to CEO Eric Gratz. Many current recycling methods result in multiple single-metal compounds that must be combined to create a new cathode material. The Battery Resourcers process precipitates a mixture of nickel, manganese and cobalt hydroxides. This mixed metal cathode precursor simplifies battery preparation and could reduce manufacturing costs.
Meanwhile, the DOE ReCell team is developing so-called direct recycling methods for recovering and reusing battery materials without expensive processing. One approach is to remove the electrolyte with supercritical carbon dioxide, then crush the cell and physically separate the components, for example on the basis of differences in density.
In principle, almost all components can be reused after this simple treatment. In particular, because the process does not use acids or other reagents, the morphology and crystal structure of the cathode materials remain intact and the materials retain the electrochemical properties that enhance them. Gaines says that more work is needed to implement this cost saving approach.

Credit: Alireza Rastegarpanah and Rustam Stolkin / Extreme Robotics Lab
At the University of Birmingham, Alireza Rastegarpanah, a member of the ReLib team, develops robotic methods for automated and safe processing of spent Li-ion batteries.
The principal investigator of the Birmingham University ReLiB project, Paul Anderson, said the team clearly saw the opportunity to enhance the economic efficiency of battery recycling through automation. To this end, the team is developing robotic procedures to sort, disbademble and recover valuable materials from Li-ion batteries. Allan Walton, a coin researcher in Birmingham, adds that the use of robotic devices to disbademble batteries would help avoid the risk of electrical and chemical injuries to human workers. Automation could also lead to improved separation of battery components, increasing their purity and value, he adds.
Although most of these strategies are still in an early stage of development, they are increasingly needed. Currently, the number of end-of-life electric vehicle batteries is low, but it is about to skyrocket. Many obstacles prevent large-scale recycling, but "opportunities always coexist with challenges," says An of Polytechnic of Hong Kong. It's time to take the bull by the horns and take the recycling of Li-ion batteries seriously.
News in chemistry and engineering
ISSN 0009-2347
Copyright © 2019 American Chemical Society
Source link