[ad_1]
A new study reported in Nature Communications has discovered activators of molecules that act as drivers for moving materials in nerve cells.
Nerve cells are the basis of the nervous system in animals, as they transfer information between the various sensory and effector organs of the body and the brain and spinal cord. However, this function relies heavily on the transport of several proteins and other molecules between different parts of the cell.
A nerve cell includes a cell body and several short branches called dendrites. It also has a long projection called an axon that keeps signals away from the neuron. The nerve cells also have tiny microtubule tubular tracks along which the cargo is moved to and from the dendrites and axons by an badociated molecular engine called KIF1C.
Human cells synthesize many types of motor molecules, called kinesins and dyneins. These transport the cargo to the opposite ends of the cell and both engines are often present on the cargo so that they can change direction if their progress is hampered.

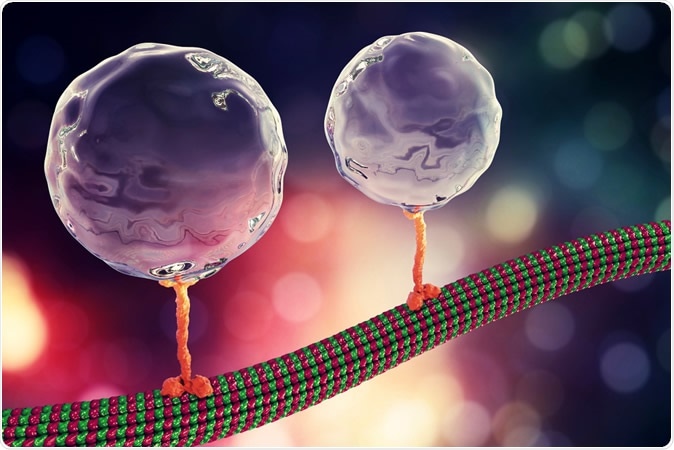
Intracellular transport, kinesin motor proteins transporting molecules moving through microtubules, 3D illustration. Kateryna Kon / Shutterstock
There are 45 kinesins, but only a few of them are really understood, in terms of what puts the motors into action. Among them, KIF1C is the fastest motor of nerve cells and is used for various processes within the neuron requiring two-way freight transport. The defects of this motor are at the root of several debilitating nervous disorders, including hereditary spastic paraplegia, which affects about 135,000 people worldwide. Alzheimer's disease and dementia are other conditions related to the malfunction of molecular motors.
KIF1C is a typical molecular motor, a protein that acts as a converter of chemical energy into mechanical energy. This helps it withstand various loads in the cell's dense core vesicles. KIF1C also carries molecules called integrins that are important for cell adhesions.
KIF1C must remain idle during the loading process and move the cargo once the loading is complete until it is actually unloaded at the end of the trip. Compared to the size of the motor, the nerve cells are incredibly long, up to 3 feet. The transport system must be both foolproof to avoid any inhibition of nerve function.
The current study at the University of Warwick aimed to unravel the activation / inactivation mechanism of KIF1C. Scientists have discovered that the inactive state of the KIF1C molecular motor is related to its self-inhibition, caused by the folding of the molecule on itself. This causes the binding of its stem to the part that attaches to the microtubules forming the tracks and keeps it out of action.
When its function is required, it uses two other proteins on the cargo, called PTNPN21 and Hook3. One or the other of these proteins binds to the stem of the KIF1C molecule, resulting in its deployment and activation. The result is that she sneaks into the tracks and starts running along them, much like a marathon runner starting the race with the choke gun.
When these proteins are present, they increase the landing rate of KIF1C on runways by approximately 40%. Thus, the load can activate the KIF1C engine when it is loaded. This may be a way that kinesins can carry cargo in both directions without drawing any contradiction. When these proteins are present, they can rescue the function of cells lacking adequate KIF1C expression.
If this finding is confirmed, it could be used to start or increase motor activity to displace the required cargo in neurons to normalize functioning in KIF1C-depleted nerve cell disorders. The team is currently considering working on hereditary spastic paraplegia.
However, there is still a vast area of research on the regulation of KIF1C in space and time inside cells, to allow its application to many neurological disorders. Anne Straube, researcher, said, "If we understand how engines are powered off, we may be able to design cellular transport machines with modified properties. These could potentially be transferred to patients with cellular defect transport to compensate for defects. They can also be used in nanotechnology to build new materials by exploiting their ability to concentrate enzymes or chemical reagents. We also study the properties of engines with mutations of patients to understand why they work less well. "
Journal reference:
PTPN21 and Hook3 Relieve Self-Inhibition of KIF1C and Activate Intracellular Transport, Nida Siddiqui, Alexander James Zwetsloot, Alice Bachmann, Daniel Roth, Hamid Hussain, Jonathan Brandt, Irina Kaverina and Anne Straube, Nature Communications Volume 10, Issue of the article: 2693 (2019) 10.1038 / s41467-019-10644-9, https://www.nature.com/articles/s41467-019-10644-9
[ad_2]
Source link