.jpg)
[ad_1]
Researchers in the United States have shown that targeting the N-terminal domain (NTD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral spike protein could provide broad neutralization of the variants of concern.
The SARS-CoV-2 virus is the causative agent of the current 2019 coronavirus disease (COVID-19) pandemic, and the spike protein is the main structure the virus uses to bind to and infect host cells. . This peak is the primary target for neutralizing antibodies after infection with SARS-CoV-2 and, therefore, the target of most vaccine design approaches.
The team – from the Icahn School of Medicine in Mount Sinai in New York and the Washington University School of Medicine in Missouri – say the findings could inform the design of next-generation vaccines that will protect against both SARS-CoV -2 currently in circulation and the new SARS-CoV-2. worrisome variants.
A pre-printed version of the research paper is available on the website bioRxiv* server, while the article is subject to peer review.
.jpg)
Researchers are trying to better understand vaccine-induced immune responses
As safe and effective COVID-19 vaccines are developed and deployed in record time, researchers urgently try to better understand vaccine-induced immune responses, the broadly neutralizing epitopes that are targeted, and the effectiveness of those epitopes. against new epitopes and potentially more transmissible viral variants.
Understanding the immunodominance of the spike protein at a structural level would help identify requirements for broader antibody responses against SARS-CoV-2 and inform the development of next-generation vaccines.
The viral spike glycoprotein mediates the infection process when its receptor binding domain (RBD) attaches to the host cell receptor angiotensin 2 converting enzyme (ACE2).
Antibody responses to the spike protein in serum, the memory B cell compartment, and mucosal surfaces have already been well characterized in terms of kinetics, binding specificity, and neutralizing power.
“The peak serum anti-SARS-CoV-2 antibody titers after natural infection are variable, may decrease to some extent over time, and have suboptimal neutralizing activity against newer viral variants, although ‘they are protective,’ writes Goran Bajic and his colleagues.
What did the researchers do?
Antibodies derived from memory B cells target both unique and overlapping epitopes that contribute to the polyclonal epitope coverage of the spike protein and preserve binding to viral variants of concern.
Bajic and his colleagues studied the first events involved in B cell activation after COVID-19 vaccination to structurally profile new antibody epitopes.
The team had previously isolated and characterized antibodies derived from the plasmablast of Pfizer-BioNTech BNT162b231 vaccine recipients.
The researchers observed that the overall neutralizing antibody responses were directed not only towards the RBD peak but also towards the NTD, suggesting a co-dominance of these two domains.
“Since NTD appears to be an important component of vaccine-induced responses, we wanted to deepen our understanding of the neutralizing epitopes in this region of the SARS-CoV-2 peak, for which only limited structural information is currently available,” wrote Bajic and his colleagues.
The researchers therefore focused on a vaccinee who had developed a strong neutralizing antibody response to the NTD peak.
Analysis of this participant’s plasma response led to the identification of a neutralizing monoclonal antibody (mAb) called PVI.V6-14 which had heavy and light chains without somatic hypermutation.
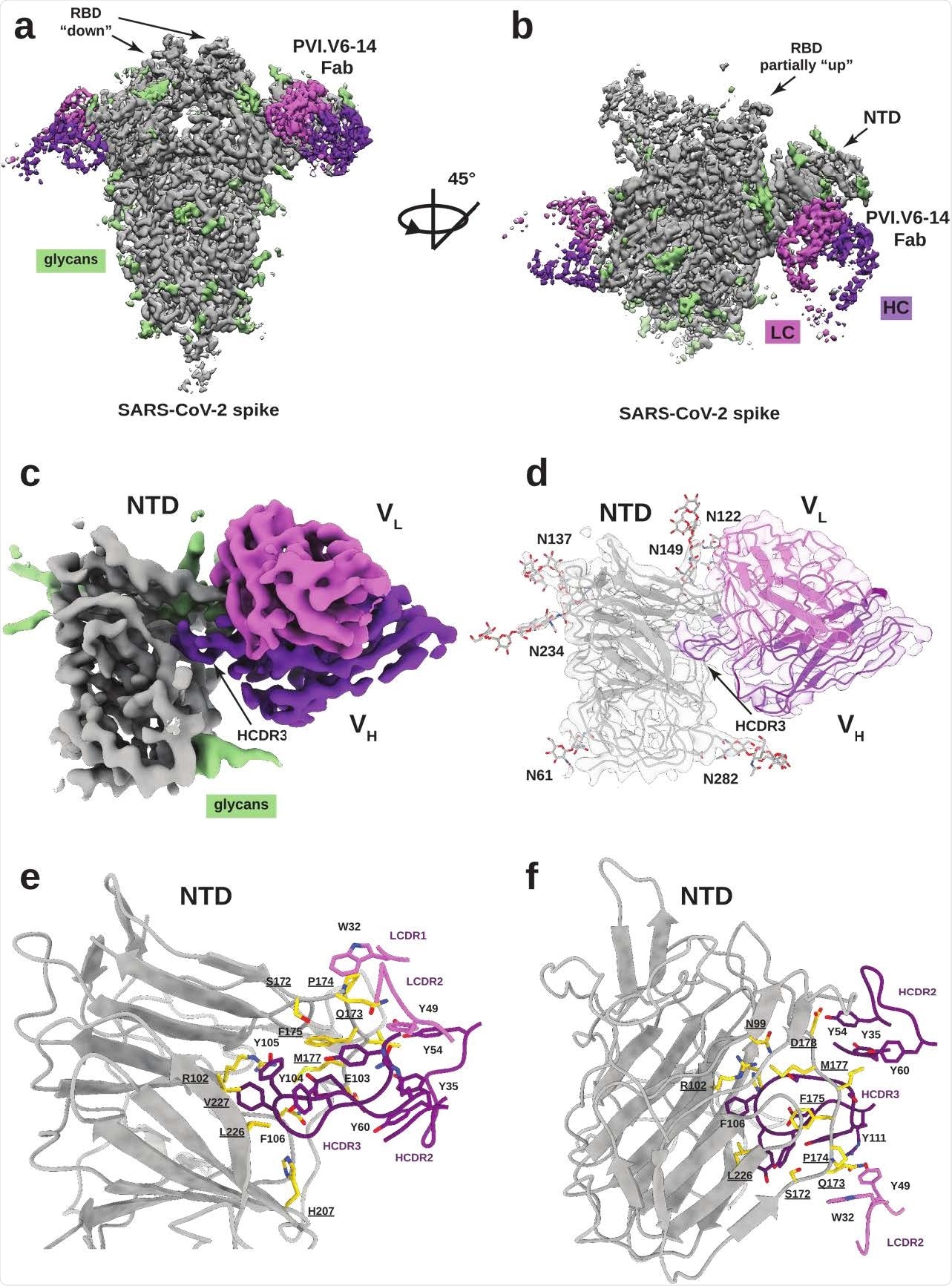
PVI.V6-14 mAb recognizes a new epitope on the SARS-CoV-2 spiked NTD. Cryo-EM reconstruction of the spiked trimer SARS-CoV-2 (gray, glycans in green) with PVI.V6-14 Fab (heavy chain in purple, light chain in pink) bound to MTN, with two views rotated 45 degrees . Two Fabs are linked, two RBDs are in “down” conformation. (c) Refined focus map of NTD-linked PVI.V6-14 at nominal resolution of 3.2 Å, with HCDR3 inserted into the hydrophobic pocket of the NTD. (d) Atomic model integrated into the cryo-EM map of the PVI.V6-14: NTD complex. (ef) Details of intermolecular interactions between PVI.V6-14 and NTD are dominated by the HCDR3 loop. Amino acid residues interacting with NTDs are shown in gold with the residue number labels underlined.
What did the current study find?
Now, using single-particle electron cryomicroscopy, researchers have determined the high-resolution structure of this mAb in complex with the spike protein SARS-CoV-2.
This revealed that PVI.V6-14 targets a previously uncharacterized neutralizing epitope on the NTD side.
The team found that PVI.V6-14 stabilized MTN by inserting its heavy complementarity-determining region 3 loop (HCDR3) into a hydrophobic pocket that was previously shown to bind to the heme metabolite, the biliverdin.
“We have discovered that mAb PVI.V6-14 belongs to an as yet undescribed class of antibodies that bind in a previously identified hydrophobic cavity to bind biliverdin,” the researchers say.
Functional binding and neutralization data showed that PVI.V6-14 directly competes with biliverdin. Additionally, due to the conserved nature of the epitope, mAb maintains binding to the B.1.1.7 (alpha) and B.1.351 (beta) variants of SARS-CoV-2 of concern.
The findings could pave the way for combination antibody therapies and next-generation vaccines
The team says the study supports the development of a cocktail of combined antibodies that targets both RBD and NTD.
“Our study illustrates the feasibility of targeting NTDs to achieve broad neutralization against SARS-CoV-2 variants,” writes Bajic and colleagues.
“Targeting NTDs offers an alternative to the design of RBD-centric vaccine immunogens and paves the way for next-generation vaccines that target NTDs in addition to RBD and potentially therapeutic antibodies that combine both mAbs neutralizers targeting RBD and NTD, ”they conclude. .
*Important Notice
bioRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link