.jpg)
[ad_1]
Italian researchers have demonstrated the effectiveness of using modified outer membrane vesicles (OMVs) as a vaccine to protect against coronavirus disease 2019 (COVID-19) in an animal model.
These OMVs are vesicles derived from bacteria that can be engineered to incorporate different viral antigens to induce potent immune responses.
Now, Guido Grandi of the University of Trento and his colleagues have shown that OMVs incorporating peptides derived from the peak protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes COVID -19 – elicit an effective immune response when administered to mice.
The vaccination induced neutralizing antibody titers sufficient to protect mice that were confronted with the original strain of SARS-CoV-2 identified in Wuhan, China (Wuhan-1 isolate).
Additionally, the team showed that OMVs can be successfully designed to induce immunity against a different variant of SARS-CoV-2.
“Overall, given the convenience associated with ease of engineering, production and distribution, our results demonstrate that OMV-based SARS-CoV-2 vaccines can be a critical addition to vaccines today. available, ”writes Guido Grandi of the University of Trento and colleagues.
A pre-printed version of the research paper is available on the website bioRxiv* server, while the article is subject to peer review.
.jpg)
Learn more about the vaccines available to date
The rapid deployment of immunization protecting against SARS-CoV-2 infection represents the most promising approach to tackle the COVID-19 pandemic.
Given the continued evolution of SARS-CoV-2 and the emergence of multiple variants of concern, an effective long-term strategy where vaccines are updated based on the extent of antigen drift is likely to be effective. be crucial.
More than 280 vaccine candidates are currently in various stages of development, and more than 100 are currently being evaluated in phase 2 clinical trials.
While protein-based vaccines form the most important part of these products, messenger RNA (mRNA) and viral vector vaccines were the first to receive emergency use authorization for deployment. mass in the world.
“As a result, the world has witnessed an unprecedented technical and economic challenge, given the need to manufacture, distribute, store and administer billions of doses of vaccine in every country,” write Grandi and his colleagues.
To overcome these challenges, vaccine production must use easily scalable and inexpensive processes to ensure that vaccination is available in all countries, regardless of economic and climatic conditions.
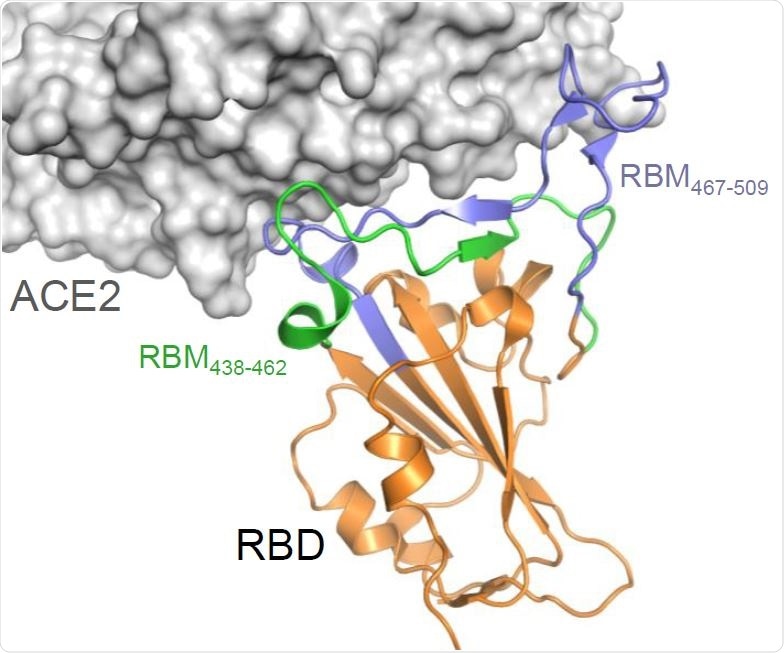
Construction and production of OMV carrying the SARS-CoV-2 RBM antigens (A) Topology of the interaction between SARS-CoV-2 RBD and ACE2 with the indication of the two RBM polypeptides tested in this study.
Where do OMVs come from?
In recent years, OMVs have emerged as an attractive tool capable of coupling excellent integrated adjuvanticity with an easily scalable production and purification process.
Importantly, in addition to the simple and cost-effective setup required to produce and purify OMVs, the antigen-decorated vesicles are extremely stable for long-term storage at room temperature, making them a convenient vaccine to distribute anywhere in the world. world. “, says Grandi and his colleagues.
What did the researchers do?
Researchers designed OMVs that incorporated peptides from the receptor binding motif (RBM) of the SARS-CoV-2 spike protein. This RBM tip forms the interface with the angiotensin-converting enzyme 2 (ACE2) of the human host cell receptor during the initial phase of the infection process.
When the team administered OMVs to CD1 mice, all sera collected from the animals contained high levels of RBM-specific immunoglobulin G (IgG) antibodies.
To test whether the antibodies could also neutralize the SARS-CoV-2 infection in vitro, the team performed a neutralization test using a pseudotyped lentiviral vector with a spike protein derived from the Wuhan-1 isolate.
Researchers found that OMVs effectively neutralized these SARS-CoV-2 spiked pseudotyped vectors.
The vaccine protected mice infected with SARS-CoV-2
To test the protective effect of the vaccine in vivo, the team immunized eight K18-hACE2 transgenic mice with a main dose of 10 µg of OMV or phosphate buffered saline (PBS), followed by a second dose of OMV or PBS 14 days later. They then infected the mice intranasally with SARS-CoV-2.
By the fourth day after infection, mice treated with PBS had developed severe disease, while mice treated with OMV developed much milder disease.
When the team tested samples taken from the lungs of animals five days after infection, they found that viral RNA and replicating virus could only be detected in one of four immunized mice, indicating the ability of the vaccine to prevent viral replication in the respiratory tract. leaflet.
Finally, the researchers designed OMVs incorporating RBM peptides derived from a different genetic variant of SARS-CoV-2. When the mice were vaccinated with these OMVs, an equally potent neutralizing activity was observed.
A “promising candidate” for vaccination against SARS-CoV-2
Grandi and his colleagues say that the immunity induced by the OMV-based vaccine is sufficient to protect K18-hACE2 transgenic mice from intranasal challenge with SARS-CoV-2.
“Considering the vaccine’s efficacy in the animal model, the ease of its engineering, the cost-effective production process and the stability at room temperature, we are proposing the OMV-based vaccine as a promising candidate to continue the campaign. vaccination against SARS -CoV-2 “, they conclude.
*Important Notice
bioRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link