[ad_1]
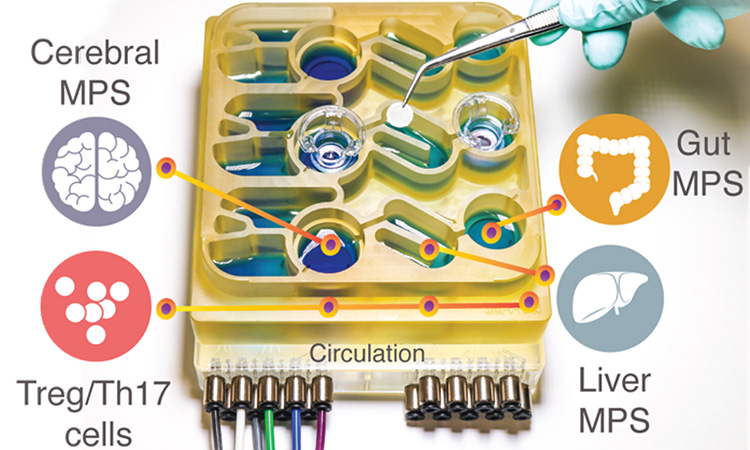
One team used their new organ-on-a-chip system to mimic interactions between the brain, liver, and colon.

The organ-on-chip system [credit: Martin Trapecar/MIT].
To help researchers better understand the gut-brain axis, the researchers developed a “organs-sure-a chip” system who replies ininteractions between the brain, liver and colon. The organoid system was developed at MIT, USA.
Use this system, the the researchers were able to model the influency that microbes live in the instinct have sure both healthy brain tissue and tissue samples from patients with Parkinson’s disease. They found that short-chain fatty acids, produced by microbes in the intestine and are transported to the brain, can have very different effects sure healthy and diseased brain cells.
“While short chain fatty acids are largely beneficial for Human health, we observed that under certain conditions they can further worsen certain brain pathologies, such as protein misfolding and neuronal death, linked to Parkinson’s disease, ”said Martin Trapecar, postdoc at MIT and senior author of the study.
For several years, researchers have been developing systems – small devices that can be used to grow engineered tissue models of different organs, connected by microfluidic channels. They found that short chain fatty acids (SCFAs), molecules produced by microbes in the intestine, can make autoimmune diseases worse ininflammation associated with ulcerative colitis in certain conditions. SCFA, which inincluding butyrate, propionate and acetate, may also have beneficial effects on tissues, including inincreased immune tolerance.
Previous research has found a link between SCFAs and Parkinson’s disease in mouse. SCFAs, which are produced by bacteria as theand consumes undigested fiber in the instinct, accelerated the progression of the disease, while the mice raised in a germ-free environment grows more slowly the disease.
Credit: Martin Trapecar / MIT.
The team decided to explore these results further, using themicrophysiological model ir. In the new study, the scientists added the brain and circulating immune cells theand multiorgan system. The cells that the MIT researchers used to their The Parkinson’s model carries a mutation that causes a protein called alpha synuclein to build up, which damages neurons and causes ininflammation in brain cells. The researchers generated brain cells that have this mutation corrected but are otherwise genetically identical and of the same patient as the diseased cells.
They studied these two sets of brain cells in microphysiology systems that were not connected to any other tissue and found that the Parkinson’s cells showed more ininflammation that the healthy and corrected cells. The Parkinson’s cells were also deficient in their ability to metabolize lipids and cholesterol.
The researchers then connected the brain cells to tissue models of the the colon and liver, using channels that allow immune cells and nutrients, inincluding SCFA, to circulate between themr. They found that for healthy brain cells, exposure to SCFAs is beneficial and helps them to mature. How? ‘Or’ Whatnever, when brain cells from patients with Parkinson’s disease were exposed to SCFAs, the the beneficial effects have disappeared. Insquare, the cells experienced higher levels of protein misfolding and cell death. TheThese effects were seen even when immune cells were eliminated. the system, leading the researchers hypothesize that the the effects are mediated by changes in lipid metabolism.
“It appears that short chain fatty acids may be linked to neurodegenerative effects diseases by affecting lipid metabolism rather than directly affecting a certain population of immune cells, ”Trapecar said. “Now the the goal for us is to try to figure this out. “
The the researchers also plan to model other types of neurological diseases that may be influenced by the gut microbiome using their organ-on-a-chip system. The the results support the idea that Human tissue models could give intraining that animal models cannot. Professor Linda Griffith, one of the main authors of the article, is now working sure a new version of the model that fits ininclude the micro blood vessels connecting different types of tissues, allowing researchers to study How? ‘Or’ What the blood flow between the tissues influences them.
“We should really push the development of these, because it is important to start bringing more Human Characteristics into our models, ”Griffith said. “We were able to start getting information about the hHuman condition difficult to obtain mice. “
The organ-on-a-chip study was published in Scientific advances.
Source link