[ad_1]
Chicago, IL (UroToday.com) Bi-specific T-cell receptors (BiTE) are a clbad of immunotherapeutic molecules that enhance the immune response of a patient by helping to direct T cells to specific tumor cells.1 Pasotuxizumab is a BiTE that engages cells expressing the prostate-specific membrane antigen (PSMA) and CD3 epsilon subunit of the T-cell receptor complex.2 In preclinical models, pasotuxizumab prevented the growth of PC-3-huPSMA cells in NOD / SCID mice.3 The authors present here the results of the Phase 1 study on pasotuxizumab in patients with metastatic castration-resistant prostate cancer.
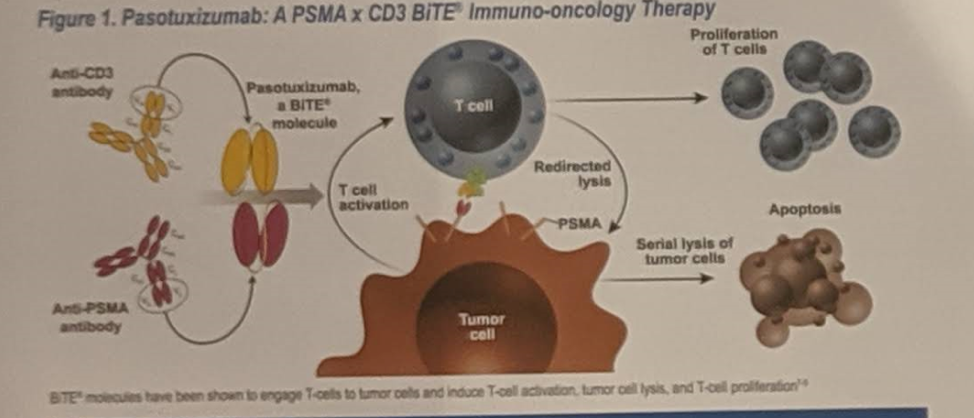
This summary provides data on 16 patients enrolled in 5 treatment cohorts. As noted below, all patients had metastatic castration-resistant prostate cancer (mCRPC) refractory to standard therapy.
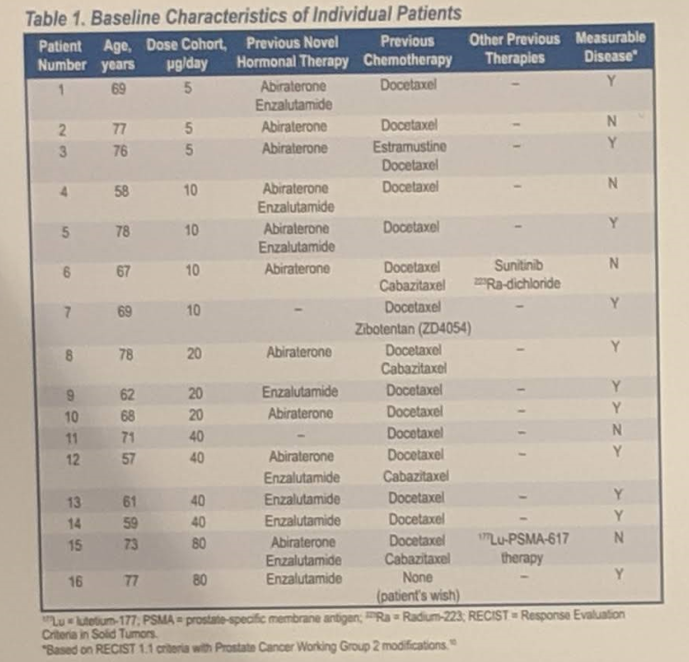
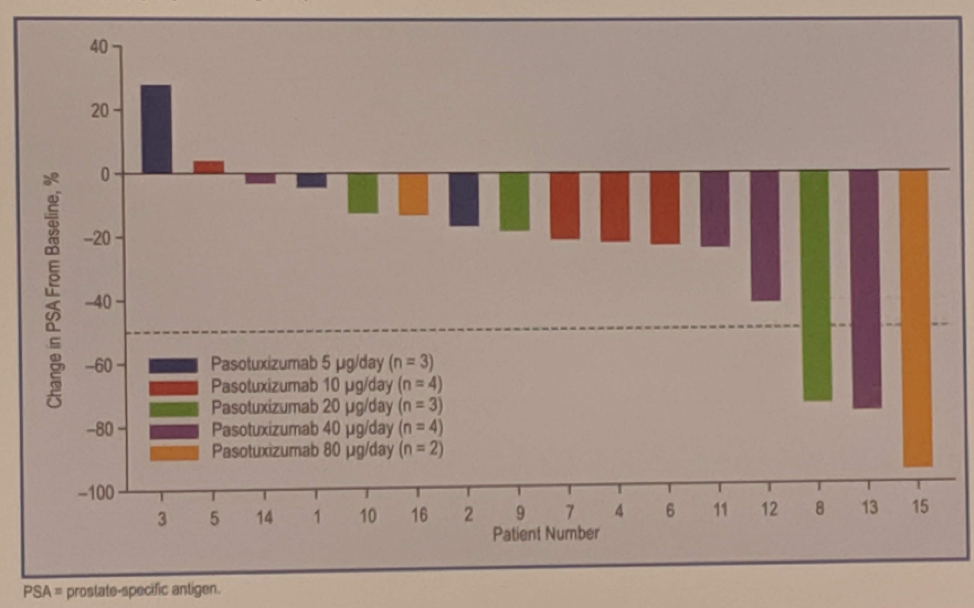
Antitumor activity was found to be dose-dependent, with an average variation of prostate-specific antigen (PSA) of -54.9% at a dose of 80 μg / d, against + 0.74% at 5 μg / d. PSA50s were observed at dose levels of 20 μg / d, 40 μg / d and 80 μg / d, and two patients had a sustained response of PSA beyond one year (14 and 19 months).

In terms of safety, the most common side effects were fever (94%), chills (69%) and fatigue (50%). 81% of patients had Grade 3 AE – the most common Grade 3 AEs were a decrease in the number of lymphocytes and infections, both at 44%. The complete list of serious adverse events (SAEs) below is similar to that observed in the treatment with BITE in lymphoma.
Pasotuxizumab is a prostate-specific membrane antigen (PSMA) targeting BiTE. It has been shown that it was active against mCRPC in this study. Several patients presented a PSA50 and two patients presented a durable response over one year. The side effect profile mimics the same symptoms as the cytokine release syndrome, which has been observed with other BiTEs used in the treatment of hematologic malignancies, including fever and chills, felt by the majority patients. This study demonstrates that BiTE can be another effective way to provide an option for immunotherapy to mCRPC patients.
Presented by: Horst-Dieter Hummel, MD, Wuerzburg University Hospital, Germany
Written by: Jason Zhu, MD. Member of Duke University's Division of Hematology and Oncology, @TheRealJasonZhu, at the ASCO 2019 # ASCO19 Annual Meeting, May 31 to June 4, 2019, Chicago, United States
References:
- Huehls AM, Coupet TA, Sentman CL. Bispecific T cell engenders for cancer immunotherapy. Immunology and Cell Biology 2015; 93: 290-6.
- Lutterbuese R, Friedrich M., Kischel R, et al. Preclinical characterization of MT112 / BAY 2010112, a new BiTE PSMA / CD3-bispecific antibody for the treatment of prostate cancer. AACR; 2011.
- Friedrich M, Raum T, Lutterbuese R, et al. Regression of prostate cancer xenografts in humans in mice by AMG 212 / BAY2010112, a novel anti-PSMA / CD3-Bispecific BiTE antibody exhibiting cross-reactivity with non-human primate antigens. Molecular treatment of cancer 2012; 11: 2664-73.
Source link